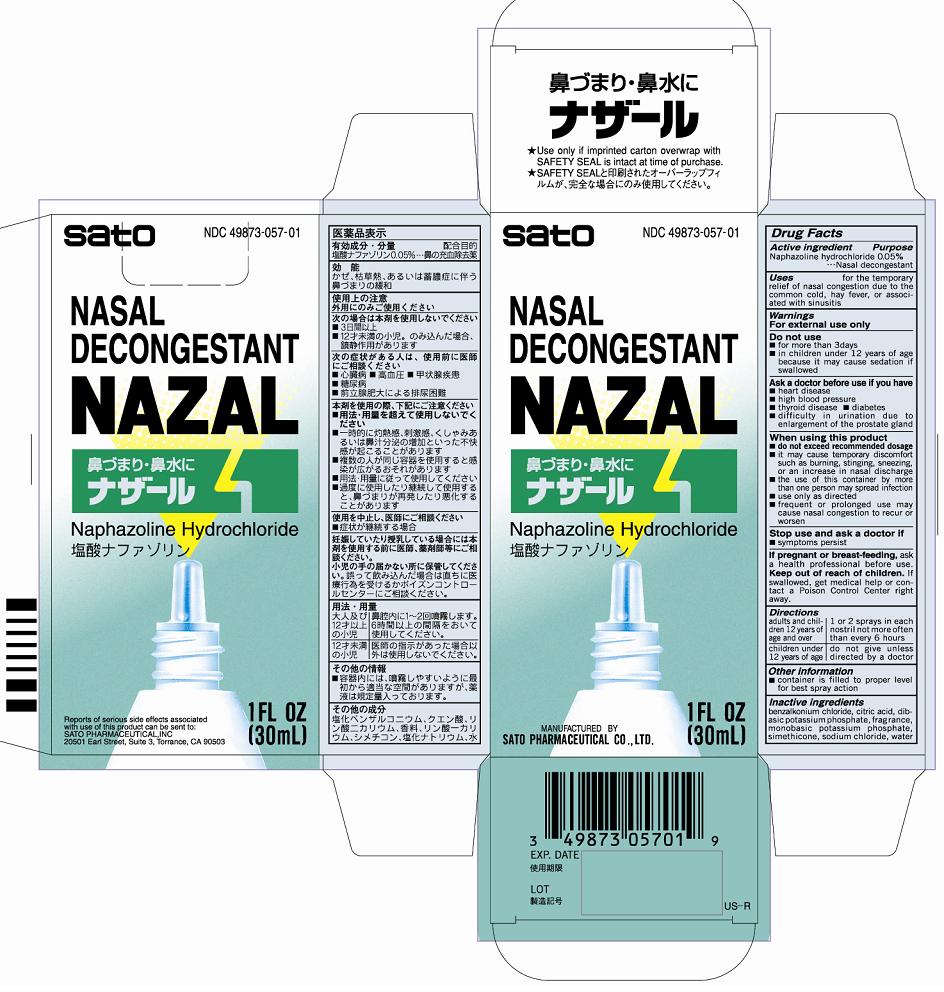
Nazal
Dosage form: liquid
Ingredients: Naphazoline hydrochloride 50mg in 100mL
Labeler: Sato Pharmaceutical Co., Ltd.
NDC code: 49873-057
Medically reviewed by Drugs.com. Last updated on Aug 18, 2025.
Active ingredients
Naphazoline hydrochloride 0.05%
Purpose Nasal decongestant
Uses for the temporary relief of nasal congestion due to the common cold, hay fever, or associated with sinusitis
Warnings
For external use only
Do not use
■for more than 3 days
■in children under 12 years of age because it may cause sedation if swallowed
Ask a doctor before use if you have
■heart disease
■high blood pressure
■thyroid disease ■diabetes
■difficulty in urination due to enlargement of the prostate gland
When using this product
■do not exceed recommended dosage
■if may cause temporary discomfort such as burning, stinging, sneezing, or an increase in nasal discharge
■the use of this container by more than one person may spread infection
■use only as directed
■frequent or prolonged use may cause nasal congestion to recur or worsen
Stop use and ask a doctor if
■symptoms persist
If pregnant or breast-feeding, ask a health professional before use.
Keep our of reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 12 years of age and older - 1 or 2 sprays in each nostril not more often than every 6 hours
Children under 12 years of age - do not give unless directed by a doctor
Other information
■container is filled to proper level for best spray action
Inactive ingredients
benzalkonium chloride, citric acid, dibasic potassium phosphate, fragrance, monobasic potassium phosphate, simethicone, sodium chloride, water
| NAZAL
naphazoline hydrochloride liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sato Pharmaceutical Co., Ltd. (690575642) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sato Pharmaceutical Co., Ltd. | 715699133 | manufacture(49873-057), label(49873-057), pack(49873-057) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
