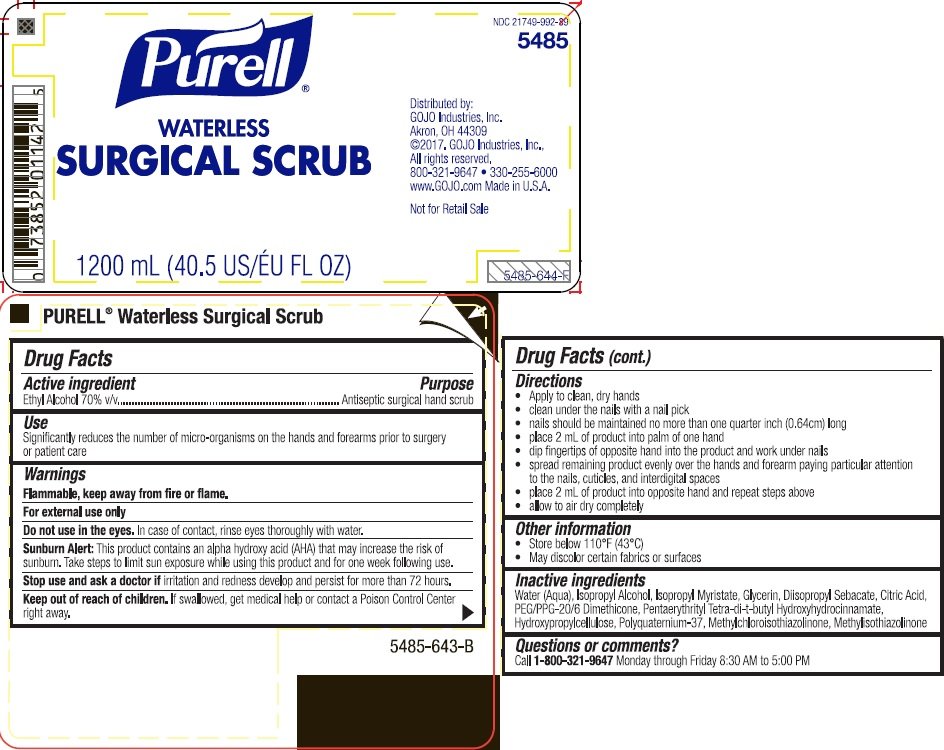
PURELL Waterless Surgical Scrub
Dosage form: liquid
Ingredients: ALCOHOL 0.7mL in 1mL
Labeler: GOJO Industries, Inc.
NDC code: 21749-992
Medically reviewed by Drugs.com. Last updated on Sep 23, 2024.
Ethyl alcohol 70% v/v
Antiseptic surgical hand scrub
Significantly reduces the number of micro-organisms on the hands and forearms prior to surgery or patient care
Flammable. Keep away from fire or flame.
For external use only
Sunburn Alert: This product contains an alpha hydroxy acid (AHA) that may increase the risk of sunburn. Take steps to limit sun exposure while using this product and for one week following use.
Do not use in the eyes. In case of contact, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation and redness develop and persist for more than 72 hours.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- clean under the nails with a nail pick
- mails should be maintained with a 1 millimeter free edge
- place 2 mL of product into palm of one hand
- dip fingers of opposite hand into the product and work under nails
- spread remaining product evenly over the hands and lower 2/3 of one forearm paying particular attention to the nails, cuticles, and interdigital spaces
- place 2 mL of product into opposite hand and repeat steps above
- allow to air dry completely
Water (Aqua), Isopropyl Myristate, Glycerin, Diisopropyl Sebacate, Citric Acid, PEG/PPG-20/6 Dimethicone, Tetradibutyl Pentaerythrityl Hydroxyhydrocinnamate, Hydroxypropylcellulose, Polyquaternium-37, Methylchloroisothiazolinone, Methylisothiazolinone
| PURELL WATERLESS SURGICAL SCRUB
alcohol liquid |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - GOJO Industries, Inc. (004162038) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| GOJO Industries, Inc. | 036424534 | MANUFACTURE(21749-992) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| GOJO Industries, Inc. | 088312414 | MANUFACTURE(21749-992), label(21749-992), pack(21749-992) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.