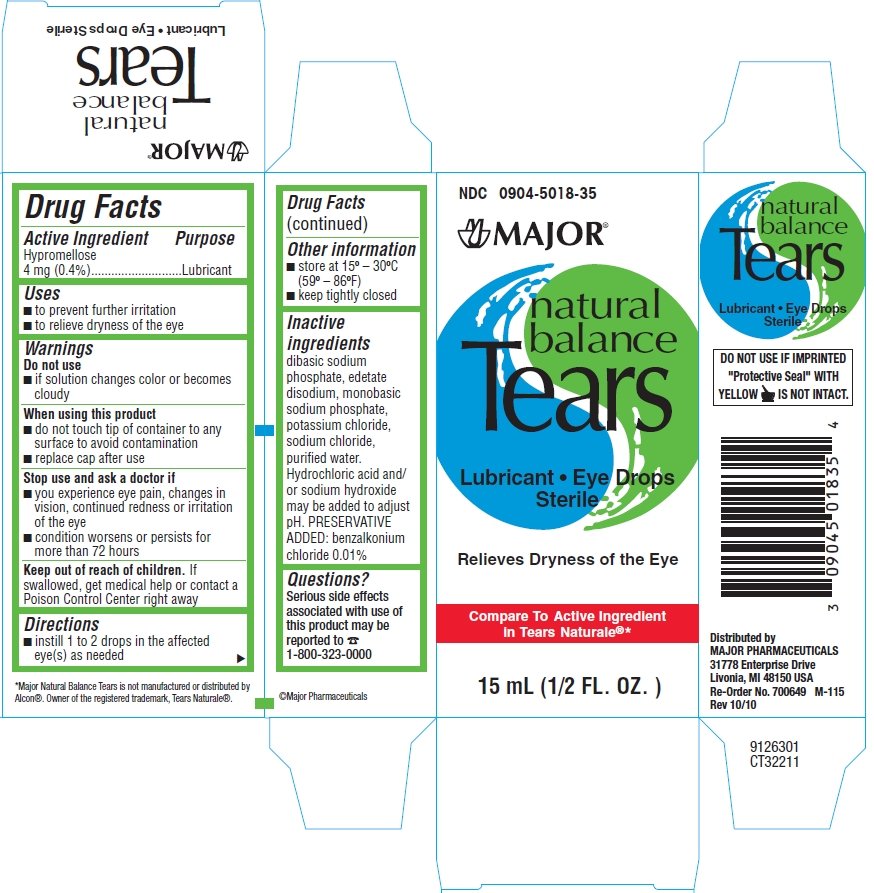
Natural Balance Tears
Dosage form: solution/ drops
Ingredients: HYPROMELLOSE 2910 (3 MPA.S) 4mg in 1mL
Labeler: Major Pharmaceuticals
NDC code: 0904-5018
Hypromellose 0.4%
Lubricant
- to prevent further irritation
to relieve dryness of the eye
Do not use if solution changes color or becomes cloudy
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after use
Stop use and ask a doctor if
- you experience eye pain, changes in vision, continued redness or irritation of the eye
- condition worsens or persists for more than 72 hours
If swallowed, get medical help or contact a Poison Control Center right away
- instill 1 to 2 drops in the affected eye(s) as needed
- store at 15° - 30°C (59° - 86°F)
- keep tightly closed
dibasic sodium phosphate, edetate disodium, monobasic sodium phosphate,
potassium chloride, sodium chloride, purified water.
Hydrochloric acid and/or sodium hydroxide may be added to adjust pH.
PRESERVATIVE ADDED: benzalkonium chloride 0.01%
Serious side effects associated with use of this product may be reported to 1800-323-0000
©Major Pharmaceuticals
| NATURAL BALANCE TEARS
hypromellose solution/ drops |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Major Pharmaceuticals (191427277) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Bausch & Lomb Incorporated | 807927397 | MANUFACTURE | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.