Dove Clinical Protection Cleartone Antiperspirant and Deodorant
Dosage form: stick
Ingredients: Aluminum Zirconium Tetrachlorohydrex GLY 20g in 100g
Labeler: Conopco Inc. d/b/a Unilever
NDC code: 64942-1204
Medically reviewed by Drugs.com. Last updated on Oct 14, 2024.
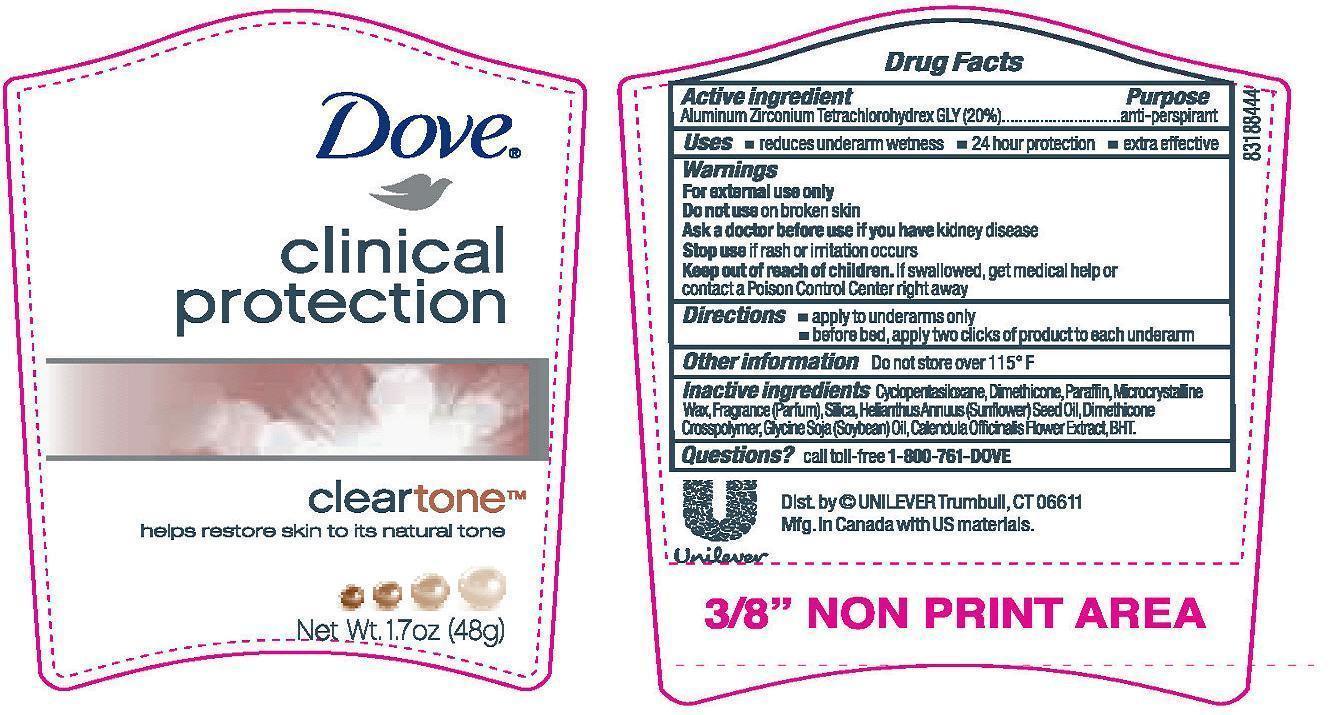
Active Ingredient
Aluminum Zirconium Tetrachlorohydrex GLY (20%)
Purpose
anti-perspirant
Uses
· reduces underarm wetness
· 24 hour protection
· extra effective
Warnings
For external use only
Do not use on broken skin
Ask a doctor before use if you have kidney disease
Stop use if rash or irritation occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
· apply to underarms only
· before bed, apply three clicks of product to each underarm
Inactive ingredients
Cyclopentasiloxane, Dimethicone, Paraffin, Microcrystalline Wax, Fragrance (Parfum), Silica, Helianthus Annuus (Sunflower) Seed Oil, Dimethicone Crosspolymer, Glycine Soja (Soybean) Oil, Calendula Officinalis Flower Extract, BHT.
Questions? Call toll-free 1-800-761-DOVE
| DOVE
CLINICAL PROTECTION CLEARTONE ANTIPERSPIRANT AND DEODORANT
aluminum zirconium tetrachlorohydrex gly stick |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Conopco Inc. d/b/a Unilever (001375088) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
