The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
wal profen cold and sinus
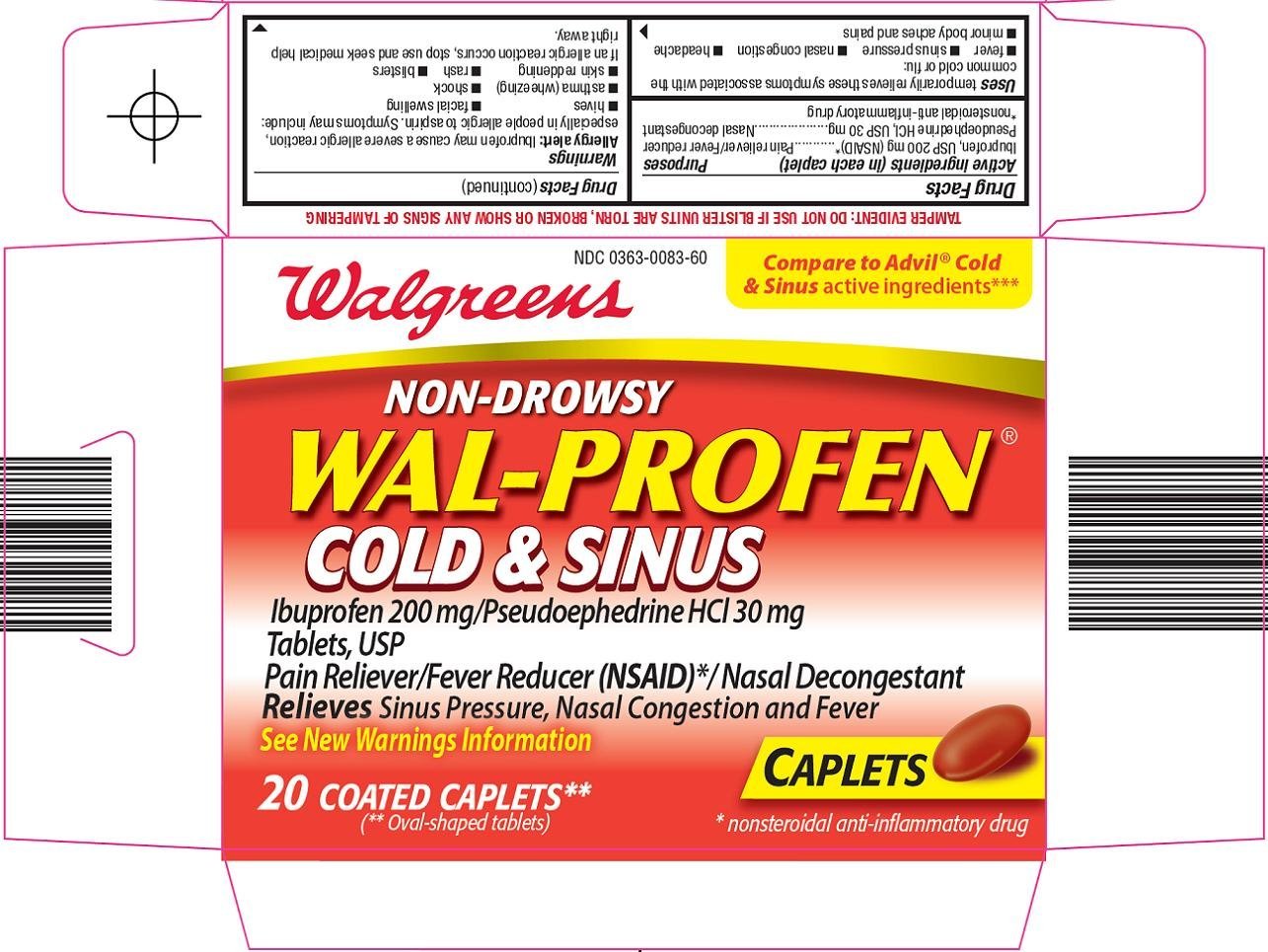
Dosage form: tablet, sugar coated
Ingredients: IBUPROFEN 200mg, PSEUDOEPHEDRINE HYDROCHLORIDE 30mg
Labeler: Walgreen Company
NDC code: 0363-0083
Ibuprofen, USP 200 mg (NSAID)*
Pseudoephedrine HCl, USP 30 mg
*nonsteroidal anti-inflammatory drug
Pain reliever/Fever reducer
Nasal decongestant
temporarily relieves these symptoms associated with the common cold or flu:
- fever
- sinus pressure
- nasal congestion
- headache
- minor body aches and pains
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- in children under 12 years of age
- right before or after heart surgery
- stomach bleeding warning applies to you
- you have problems or serious side effects from taking pain relievers or fever reducers
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, thyroid disease, diabetes or have trouble urinating due to an enlarged prostate gland
- you are taking a diuretic
- under a doctor’s care for any serious condition
- taking any other drug
- taking any other product that contains pseudoephedrine or any other nasal decongestant
- taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
- take with food or milk if stomach upset occurs
- the risk of heart attack or stroke may increase if you use more than directed or for longer than directed
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- symptoms continue or get worse
- any new symptoms appear
- you get nervous, dizzy, or sleepless
- fever gets worse or lasts more than 3 days
- nasal congestion lasts for more than 7 days
- redness or swelling is present in the painful area
ask a health professional before use. It is especially important not to use ibuprofen during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
- do not take more than directed
- the smallest effective dose should be used
- adults and children 12 years of age and over:
- take 1 caplet every 4 to 6 hours while symptoms persist. If symptoms do not respond to 1 caplet, 2 caplets may be used.
- do not use more than 6 caplets in any 24-hour period unless directed by a doctor
- children under 12 years of age: do not use
- store at 20-25°C (68-77°F). Avoid excessive heat above 40°C (104°F).
- read all warnings and directions before use. Keep carton.
acacia, calcium carbonate, carnauba wax, confectioner’s sugar, corn starch, croscarmellose sodium, crospovidone, FD&C blue no. 2 aluminum lake, FD&C red no. 40 aluminum lake, FD&C yellow no. 6 aluminum lake, gelatin, guar gum, hydrogenated vegetable oil, hydroxypropyl cellulose, kaolin, pharmaceutical ink, polyethylene glycol, powdered cellulose, povidone, pregelatinized starch, silicon dioxide, sodium benzoate, sucrose, talc, titanium dioxide, white wax
1-800-719-9260
Compare to Advil® Cold & Sinus active ingredients
NON-DROWSY
WAL-PROFEN® COLD & SINUS
Ibuprofen 200 mg/Pseudoephedrine HCl 30 mg
Tablets, USP
Pain Reliever/Fever Reducer (NSAID)*/ Nasal Decongestant
Relieves Sinus Pressure, Nasal Congestion and Fever
See New Warnings Information
CAPLETS
(**Oval-shaped tablets)
*nonsteroidal anti-inflammatory drug
| WAL PROFEN COLD AND SINUS
ibuprofen, pseudoephedrine hydrochloride tablet, sugar coated |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Walgreen Company (008965063) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.