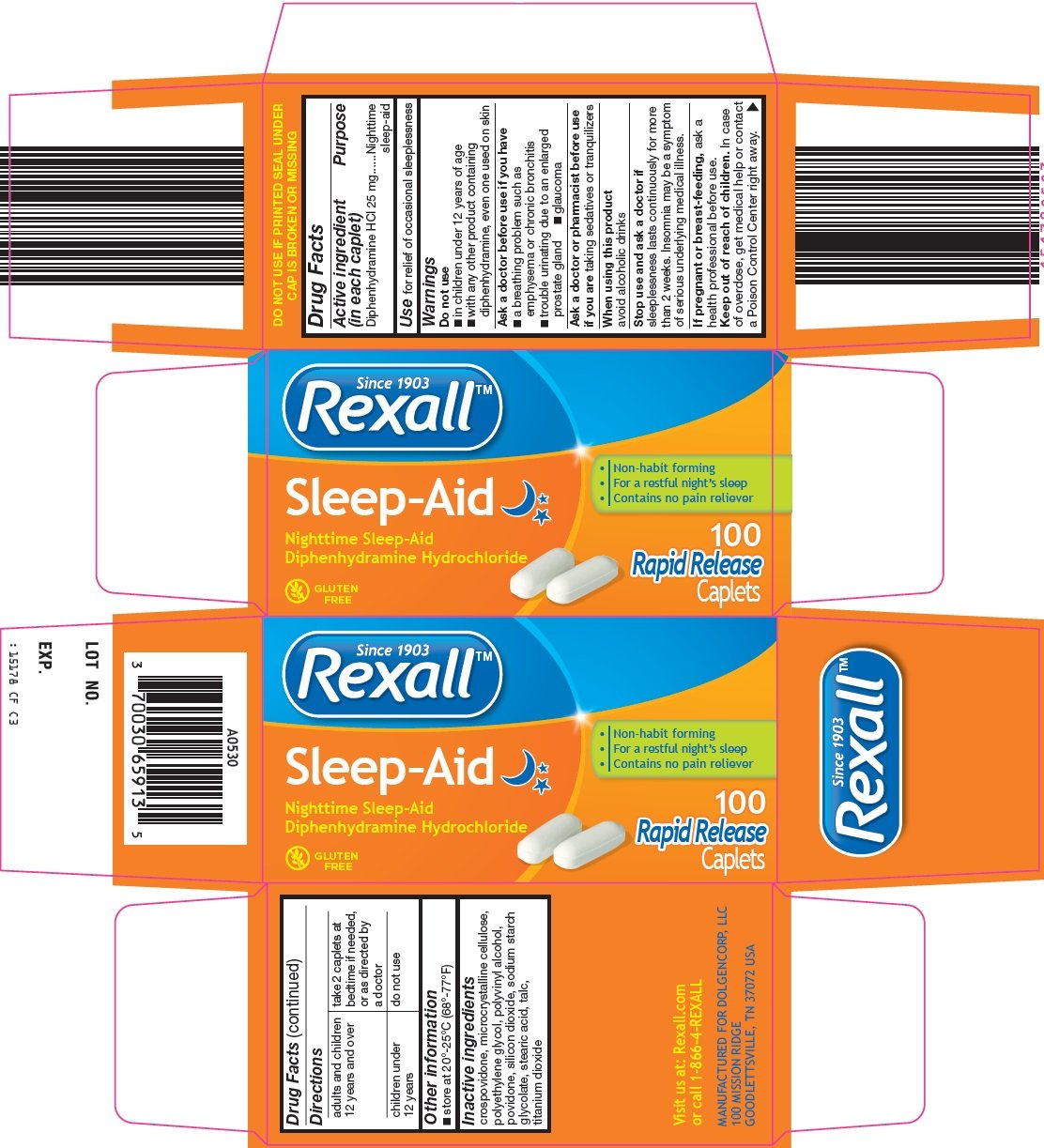
rexall sleep aid
Dosage form: tablet
Ingredients: DIPHENHYDRAMINE HYDROCHLORIDE 25mg
Labeler: Dolgencorp, LLC
NDC code: 55910-702
Medically reviewed by Drugs.com. Last updated on Nov 26, 2024.
Diphenhydramine HCl 25 mg
Nighttime sleep-aid
for relief of occasional sleeplessness
- •
- in children under 12 years of age
- •
- with any other product containing diphenhydramine, even one used on skin
- •
- a breathing problem such as emphysema or chronic bronchitis
- •
- trouble urinating due to an enlarged prostate gland
- •
- glaucoma
taking sedatives or tranquilizers
avoid alcoholic drinks
sleeplessness lasts continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
|
adults and children 12 years and over |
take 2 caplets at bedtime if needed, or as directed by a doctor |
|
children under 12 years |
do not use |
- •
- store at 20º-25ºC (68º-77ºF)
crospovidone, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, silicon dioxide, sodium starch glycolate, stearic acid, talc, titanium dioxide
Sleep-Aid
Non-habit forming
For a restful night’s sleep
Contains no pain reliever
Nighttime Sleep-Aid
Diphenhydramine Hydrochloride
100 Rapid Release Caplets
GLUTEN FREE
| REXALL SLEEP AID
diphenhydramine hydrochloride tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Dolgencorp, LLC (068331990) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
