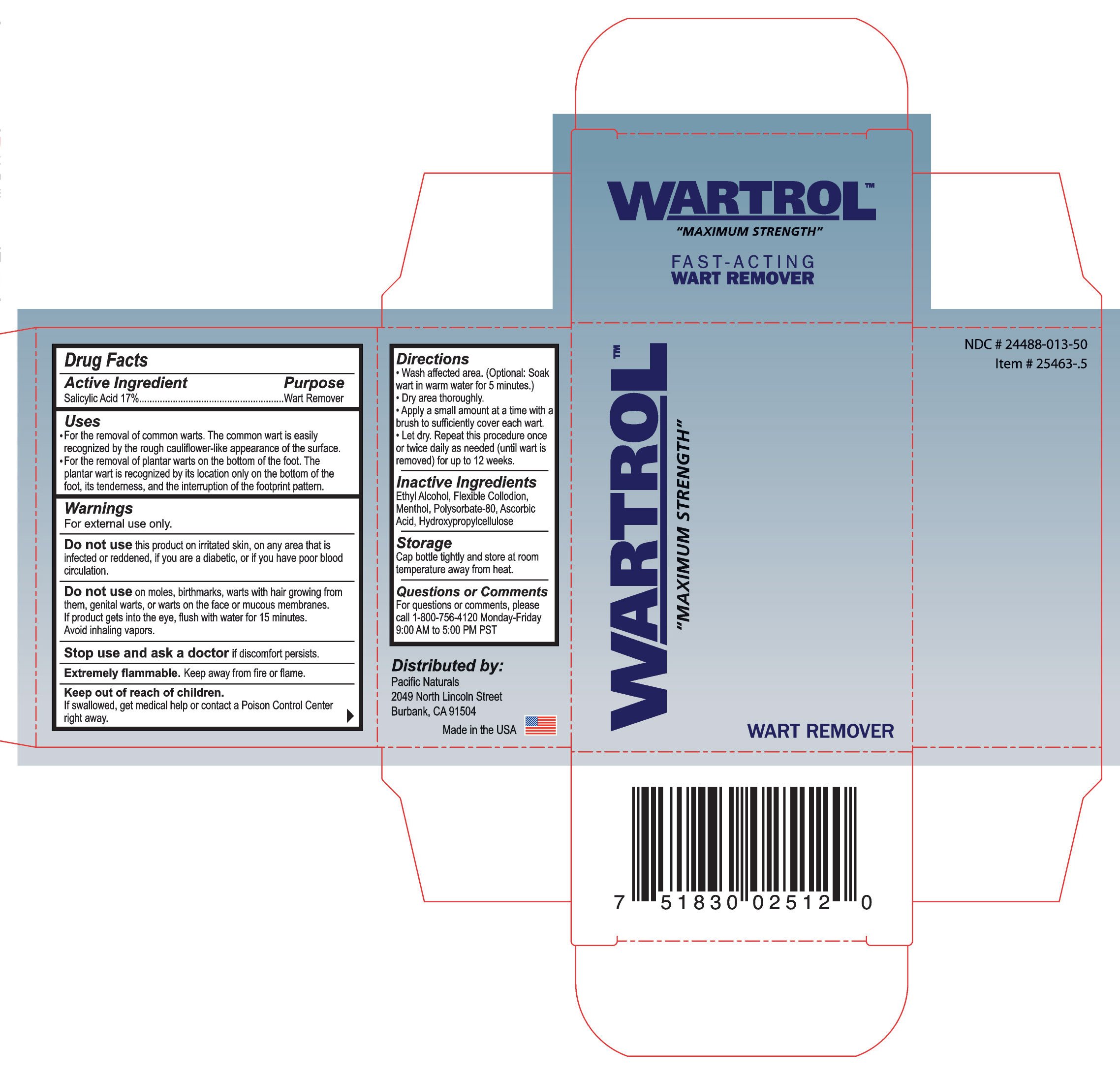
Wartrol Wart Remover
Dosage form: liquid
Ingredients: SALICYLIC ACID 2.51mL in 14.78mL
Labeler: Pacific Naturals
NDC code: 24488-013
Medically reviewed by Drugs.com. Last updated on Aug 22, 2025.
Salicylic Acid 17%
Wart Remover
- For the removal of common warts. The common wart is easily recognized by the rough cauliflower-like appearance of the surface.
- For the removal of plantar warts on the bottom of the foot. The wart is recognized by its location only on the bottom of the foot, its tenderness, and the interruption of the footprint pattern.
For external use only.
this product on irritated skin, on any area that is infected or reddened, if you are diabetic, or if you have poor blood circulation.
if discomfort persists.
Keep away from fire or flame.
If swallowed, get medical help pr contact a Poison Control Center right away.
- Wash affected area. (Optional: Soak wart in warm water for 5 minutes.)
- Dry area thoroughly.
- Apply a small amount at a time with a brush to sufficiently to cover each wart.
- Let dry. Repeat this procedure once or twice daily as needed (until wart is removed) for up to 12 weeks.
Ethyl Alcohol, Flexible Collodion, Menthol, Polysorbate-80, Ascorbic Acid, Hydroxyproylcellulose
Cap bottle tightly and store at room temperature away from heat.
| WARTROL
WART REMOVER
salicylic acid liquid |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Pacific Naturals (961956229) |
| Registrant - Creations Garden Natural Products, Inc (961956229) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Creations Garden Natural Products, Inc | 961956229 | manufacture | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.