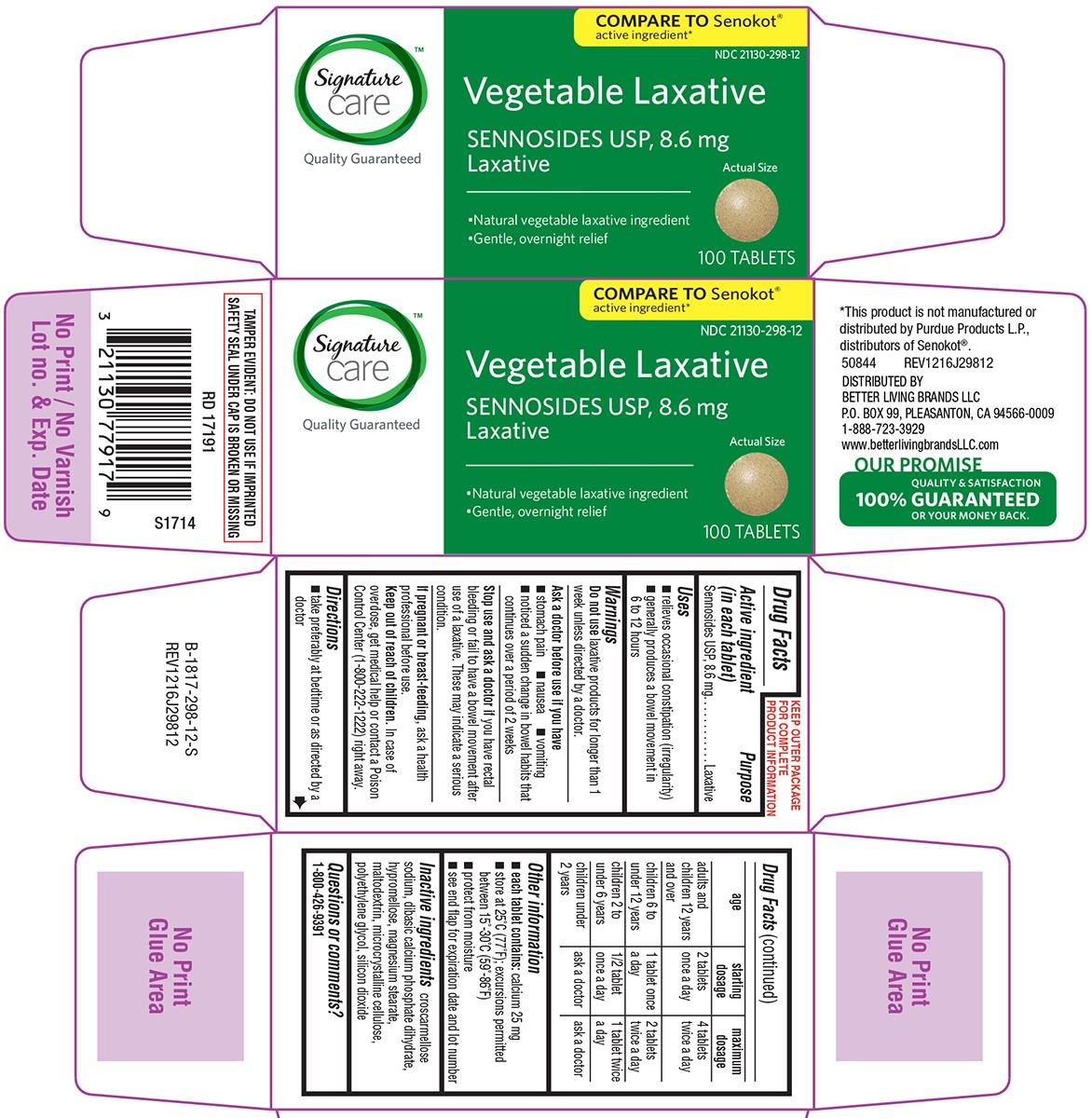
Vegetable Laxative
Dosage form: tablet
Ingredients: SENNOSIDES 8.6mg
Labeler: Better Living Brands, LLC
NDC code: 21130-298
Medically reviewed by Drugs.com. Last updated on Jun 27, 2025.
Sennosides USP, 8.6 mg
Laxative
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 6 to 12 hours
laxative products for longer than 1 week unless directed by a doctor.
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that continues over a period of 2 weeks
you have rectal bleeding or fail to have a bowel movement after use of a laxative. These may indicate a serious condition.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
- take preferably at bedtime or as directed by a doctor
| age | starting dosage | maximum dosage |
| adults and children 12 years and over | 2 tablets once a day | 4 tablets twice a day |
| children 6 to under 12 years | 1 tablet once a day | 2 tablets twice a day |
| children 2 to under 6 years | 1/2 tablet once a day | 1 tablet twice a day |
| children under 2 2 years | ask a doctor | ask a doctor |
- each tablet contains: calcium 25 mg
- store at 25ºC (77ºF); excursions permitted between 15°- 30°C (59°- 86°F)
- protect from moisture
- see end flap for expiration date and lot number
croscarmellose sodium, dibasic calcium phosphate dihydrate, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, silicon dioxide
1-800-426-9391
Signature™
care
Quality Guaranteed
COMPARE TO Senokot®
active ingredient*
NDC 21130-298-12
Vegetable Laxative
SENNOSIDES USP, 8.6 mg
Laxative
Actual Size
• Natural vegetable laxative ingredient
• Gentle, overnight relief
Actual Size
100 TABLETS
*This product is not manufactured or distributed by Purdue Products L.P., distributors of Senokot®.
50844 REV1216J29812
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
DISTRIBUTED BY
BETTER LIVING BRANDS LLC
P.O. BOX 99, PLEASANTON, CA 94566-0009
1-888-723-3929
www.betterlivingbrandsLLC.com
OUR PROMISE
QUALITY & SATISFACTION
100% GUARANTEED
OR YOUR MONEY BACK.
Signature Care 44-298
| VEGETABLE LAXATIVE
sennosides tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Better Living Brands, LLC (009137209) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867894 | MANUFACTURE(21130-298) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 038154464 | PACK(21130-298) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 967626305 | PACK(21130-298) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 868734088 | PACK(21130-298) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
