Acetaminophen Extended Release
Dosage form: tablet, film coated, extended release
Ingredients: ACETAMINOPHEN 650mg
Labeler: NCS HealthCare of KY, Inc dba Vangard Labs
NDC code: 0615-7590
Medically reviewed by Drugs.com. Last updated on Nov 18, 2024.
Active ingredient (in each caplet)
Acetaminophen 650mg
Pain reliever/fever reducer
• temporarily relieves minor aches and pains due to:
• minor pain of arthritis
• muscular aches
• backache
• premenstrual and menstrual cramps
• the common cold
• headache
• toothache
• temporarily reduces fever
Liver Warning: This product contains acetaminophen. Severe liver damage may occur if you take
• more than 6 caplets in 24 hours, which is the maximum daily amount
• with other drugs containing acetaminophen
• 3 or more alcoholic drinks every day while using this product
Acetaminophen may cause severe skin reactions. Symptoms may include:
• skin reddening
• blisters
• rash
If a skin reaction occurs, stop use and seek medical help right away.
• with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
• if you are allergic to acetaminophen or any of the inactive ingredients in this product
liver disease
taking the blood thinning drug warfarin
• pain gets worse or lasts for more than 10 days
• fever gets worse or lasts for more than 3 days
• new symptoms occur
• redness or swelling is present
These could be signs of a serious condition.
ask a health professional before use.
Overdose warning: In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222) Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
• do not take more than directed (see overdose warning)
|
Adults: |
•take 2 caplets every 8 hours with water •swallow whole; do not crush, chew, split or dissolve •do not take more than 6 caplets in 24 hours •do not use for more than 10 days unless directed by a doctor |
|
Under 18 years of age: | •ask a doctor |
• store at 20°-25°C (68°-77°F). Avoid excessive heat 40°C (104°F).
carnauba wax, colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, polysorbate 80, povidone, pregelatinized starch, stearic acid, titanium dioxide.
1-800-719-9260
U.S. Patent 7,897,172
Gluten Free
Distributed by: MAJOR® PHARMACEUTICALS
31778 Enterprise Drive, Livonia, MI 48510 USA
M-05 REV. 10/14
Acetaminophen Extended-Release Tablets 650 mg
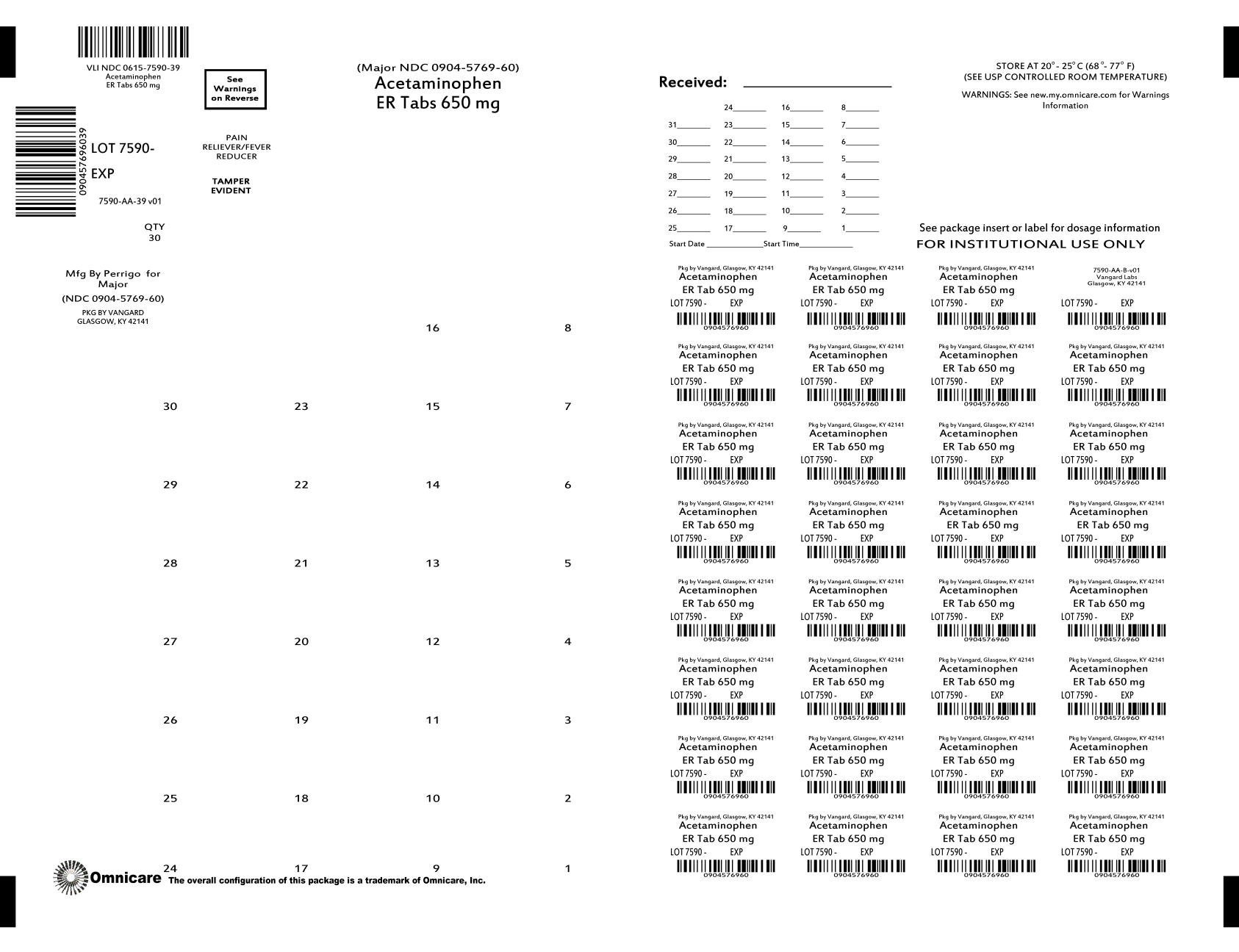
| ACETAMINOPHEN EXTENDED RELEASE
acetaminophen tablet, film coated, extended release |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - NCS HealthCare of KY, Inc dba Vangard Labs (050052943) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| NCS HealthCare of KY, Inc dba Vangard Labs | 050052943 | REPACK(0615-7590) | |
See all Acetaminophen Extended Release brands
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
