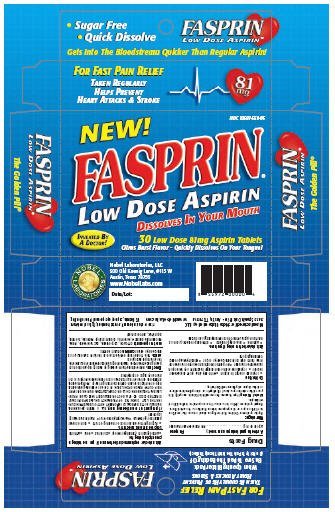
Fasprin
Dosage form: tablet, orally disintegrating
Ingredients: ASPIRIN 81mg
Labeler: Nobel Laboratories, LLC
NDC code: 10597-559
Medically reviewed by Drugs.com. Last updated on Nov 20, 2024.
Aspirin 81 mg
Pain Reliever/Fever Reducer
For temporary relief of minor aches and pains
for chicken pox or flu symptoms before a doctor is consulted about
Reye's syndrome, a rare but serious illness reported to be associated
with aspirin.
Alcohol Warning: If you consume 3 or more alcoholic drinks every day, ask
your doctor whether you should take aspirin or other pain relievers/fever
reducers. Aspirin may cause stomach bleeding.
- If you are allergic to aspirin
- With any other pain reliever/fever reducer
- If you have ever had an allergic reaction to any other pain reliever/fever reducer
- For pain for more than 10 days or for fever for more than 3 days unless directed by a doctor
- With any other product containing aspirin
- Asthma
- Bleeding problems
- Stomach problems (such as heartburn, upset stomach or stomach pain) gastric ulcers
- Anticoagulation (thinning of blood)
- Diabetes
- Gout
- Arthritis
- Ringing in the ears or loss of hearing occurs
- Pain or fever persists or gets worse
- New symptoms occur
- Redness or swelling is present
ask a health professional before use. IT IS ESPECIALLY IMPORTANT NOT TO USE ASPIRIN DURING THE LAST 3 MONTHS OF PREGNANCY UNLESS SPECIFICALLY DIRECTED TO DO SO BY A DOCTOR BECAUSE IT MAY CAUSE PROBLEMS IN THE UNBORN CHILD OR COMPLICATIONS DURING DELIVERY.
KEEP OUT OF REACH OF CHILDREN. In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions: Remove tablet from backing by bending at perforation and
peeling back cover. Place tablet on tongue and allow saliva to dissolve the
tablet for rapid absorption.
Adults: Take 1 tablet daily, do not exceed 36 tablets in 24 hours, or as
directed by a doctor. Children: Consult a doctor.
Citric Acid, Crospovidone, D and C Yellow #10, Flavor, Glucosamine Sulfate-K, Mannitol, Sodium Stearyl Fumarate, Sorbitol, Sunnette K, Zinc Gluconate
Manufactured for: Nobel Laboratories, LLC
3413 Spanish Oak Drive Austin, TX 787314www.NobelLabs.com
Store Product Below 90 degrees F. Protect from heat, light and moisture.
U.S Patents 6,596,708 / 6,930,099 and Patents Pending| FASPRIN
aspirin tablet, orally disintegrating |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Nobel Laboratories, LLC (022735189) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Avema Pharma Solutions | 804087794 | manufacture | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.