Mane n Tail Daily Control Anti-Dandruff
Dosage form: shampoo, suspension
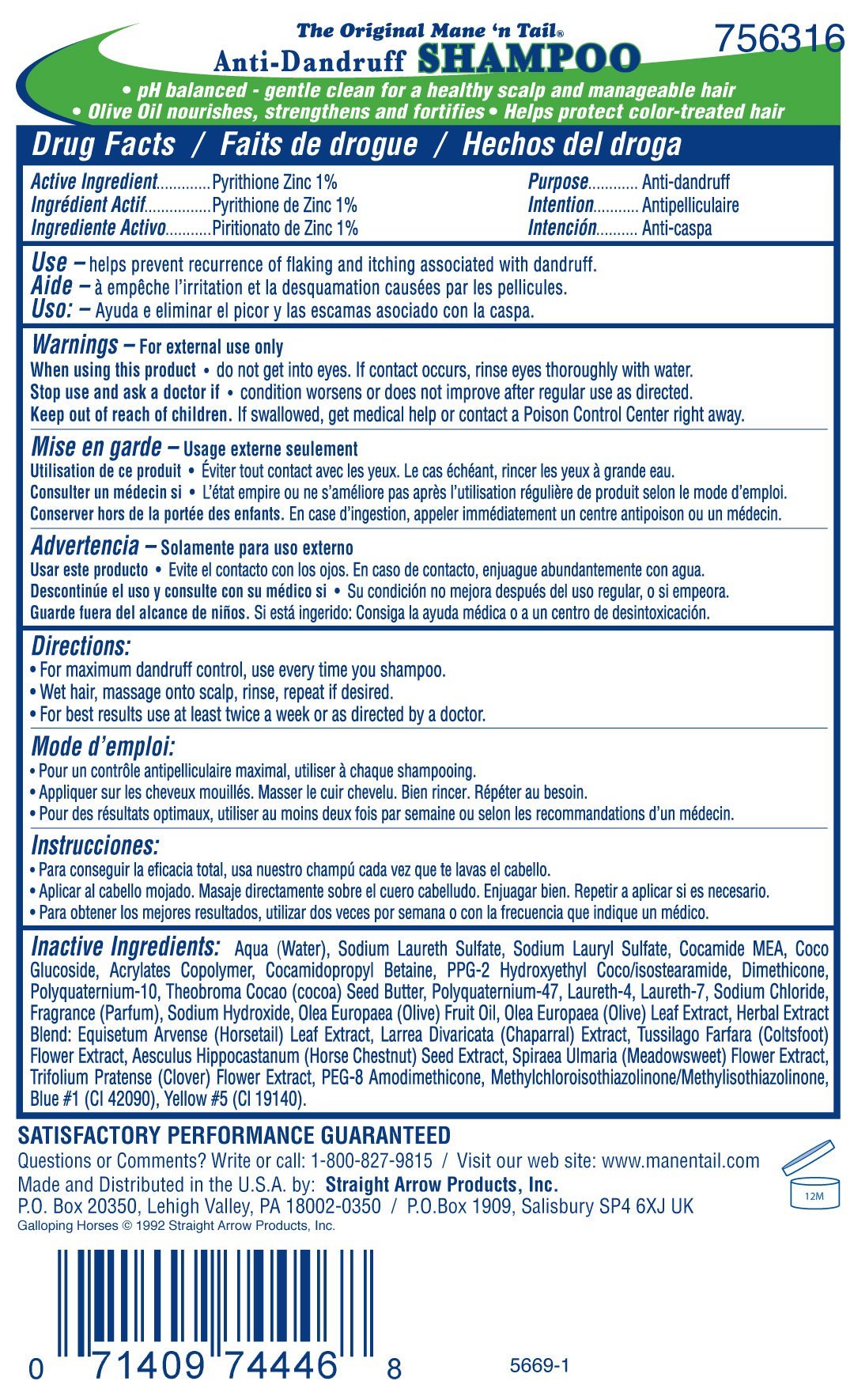
Ingredients: PYRITHIONE ZINC .01g in 1g
Labeler: Straight Arrow Products, Inc.
NDC code: 62001-0319
Medically reviewed by Drugs.com. Last updated on Sep 25, 2024.
Pyrithione Zinc 1%
Anti-dandruff
Use -helps prevent recurrence of flaking and itching associated with dandruff.
Warnings - For external use only
- do not get into eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if
- if condition worsens or does not improve after regular use as directed.
Directions:
- For maximum dandruff control, use every time you shampoo.
- Wet hair, massage onto scalp, rinse, repeat if desired.
- For best results, use at least twice a week or as directed by a doctor.
Inactive Ingredients: Aqua (Water), Sodium Laureth Sulfate, Sodium Lauryl Sulfate, Cocamide MEA, Coco Glucoside, Acrylates Copolymer, Cocamidopropyl Betaine, Fragrance (Parfum), Sodium Hydroxide, Olea Europaea (Olive) Fruit Oil, Olea Europaea (Olive) Leaf Extract, Herbal Extract Blend: Equisetum Arvense (Horsetail) Leaf Extract, Larrea Divaricata (Chaparral) Extract, Tussilago Farfara (Coltsfoot) Flower Extract, Aesculus Hippocastanum (Horse Chestnut) Seed Extract, Spiraea Ulmaria (Meadowsweet) Flower Extract, Trifolium Pratense (Clover) Flower Extract, PEG-8 Amodimethicone, Methylchloroisothiazolinone/Methylisothiazolinone, Blue #1 (CI 42090), Yellow #5 (CI 19140).

| MANE N TAIL DAILY CONTROL ANTI-DANDRUFF
pyrithione zinc shampoo, suspension |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Straight Arrow Products, Inc. (061580593) |
| Registrant - Straight Arrow Products, Inc. (061580593) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Straight Arrow Products, Inc. | 061580593 | manufacture | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
