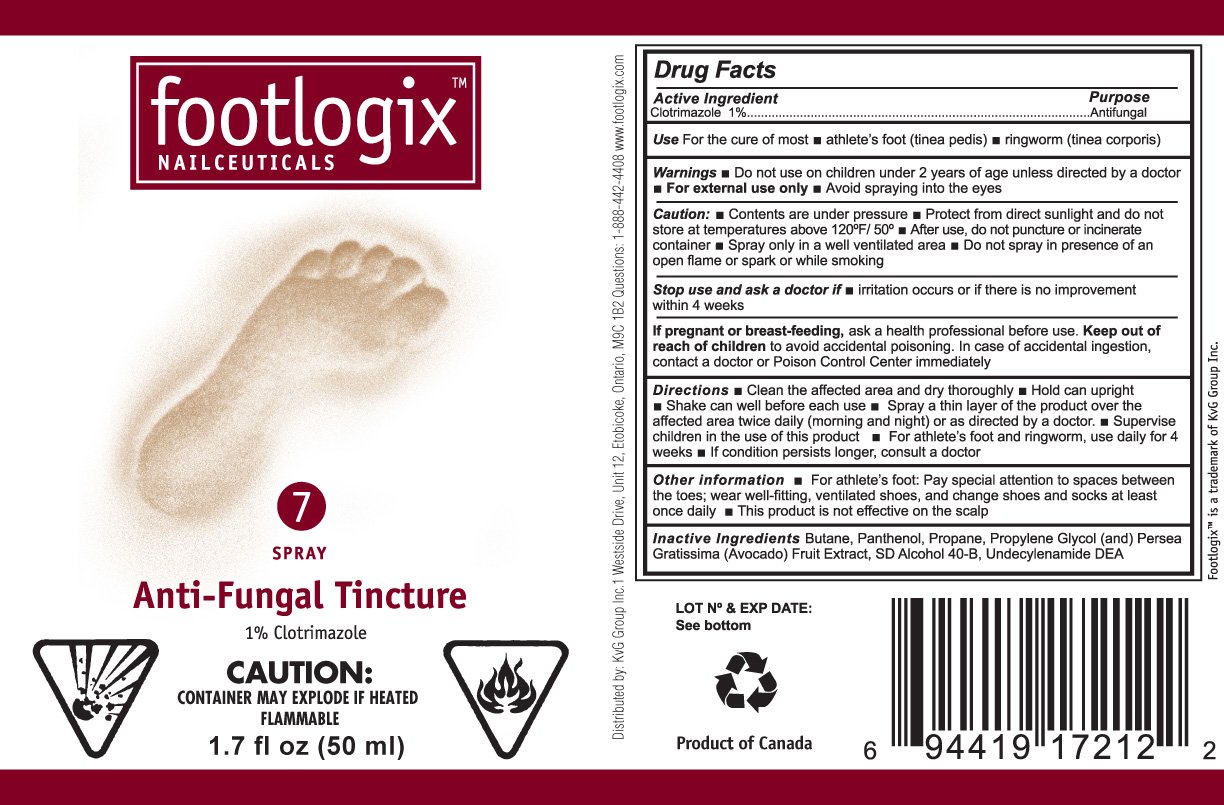
footlogix
Dosage form: aerosol, spray
Ingredients: CLOTRIMAZOLE 1mL in 100mL
Labeler: KVG Group Inc
NDC code: 42479-212
Medically reviewed by Drugs.com. Last updated on Aug 11, 2025.
Active Ingredient
Clotrimazole 1%
Clotrimazole 1%
Purpose
Antifungal
Antifungal
Use
- For the cure of most athlete’s foot (tinea pedis)
- ringworm (tinea corporis)
Warnings
- For external use only
- Do not use on children under 2 years of age unless directed by a doctor
- For external use only
- Avoid spraying into the eyes
Caution:
- Contents are under pressure
- Protect from direct sunlight and do not store at temperatures above 120F/50C
- After use, do not puncture or incinerate container
- Spray only in a well ventilated area
- Do not spray in presence of open flame or spark or while smoking
Stop use and ask a doctor if
- irritation occurs or if there is no improvement within 4 weeks
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children to avoid accidental poisoning. In case of accidental ingestion, contact a doctor or Poison Control Center immediately.
Directions
- Clean the affected area and dry thoroughly
- Hold can upright
- Shake can well before each use
- Spray a thin layer of the product over the affected area twice daily (morning and night) or as directed by a doctor.
- Supervise children in the use of this product
-
For athlete’s foot and ringworm, use
daily for 4 weeks
- If condition persists longer, consult a doctor
Other information
- For athlete’s foot Pay special attention to spaces between the toes; wear well-fitting, ventilated shoes, and change shoes and socks at least once daily.
- This product is not effective on the scalp
Inactive Ingredients Butane, Panthenol, Propylene Glycol, (and) Persea Gratissima (Avocado) Fruit Extract, SD Alcohol 40-B, Undecylenamide DEA
| FOOTLOGIX
clotrimazole aerosol, spray |
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
| Labeler - KVG Group Inc (206932605) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Assured Packaging Inc | 248916165 | manufacture | |
Revised: 08/2010
Document Id: 1e50c0d6-debd-4201-bae3-d33a3b646457
Set id: d1e56f5f-3665-420c-9753-6c1c38ba112f
Version: 1
Effective Time: 20100819
KVG Group Inc
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.