Scarguard MD
Dosage form: liquid
Ingredients: Hydrocortisone 5mg in 1mL, Phenyl Trimethicone 12mL in 100mL
Labeler: Scarguard Labs, LLC
NDC code: 64269-9903
Medically reviewed by Drugs.com. Last updated on Jun 2, 2025.
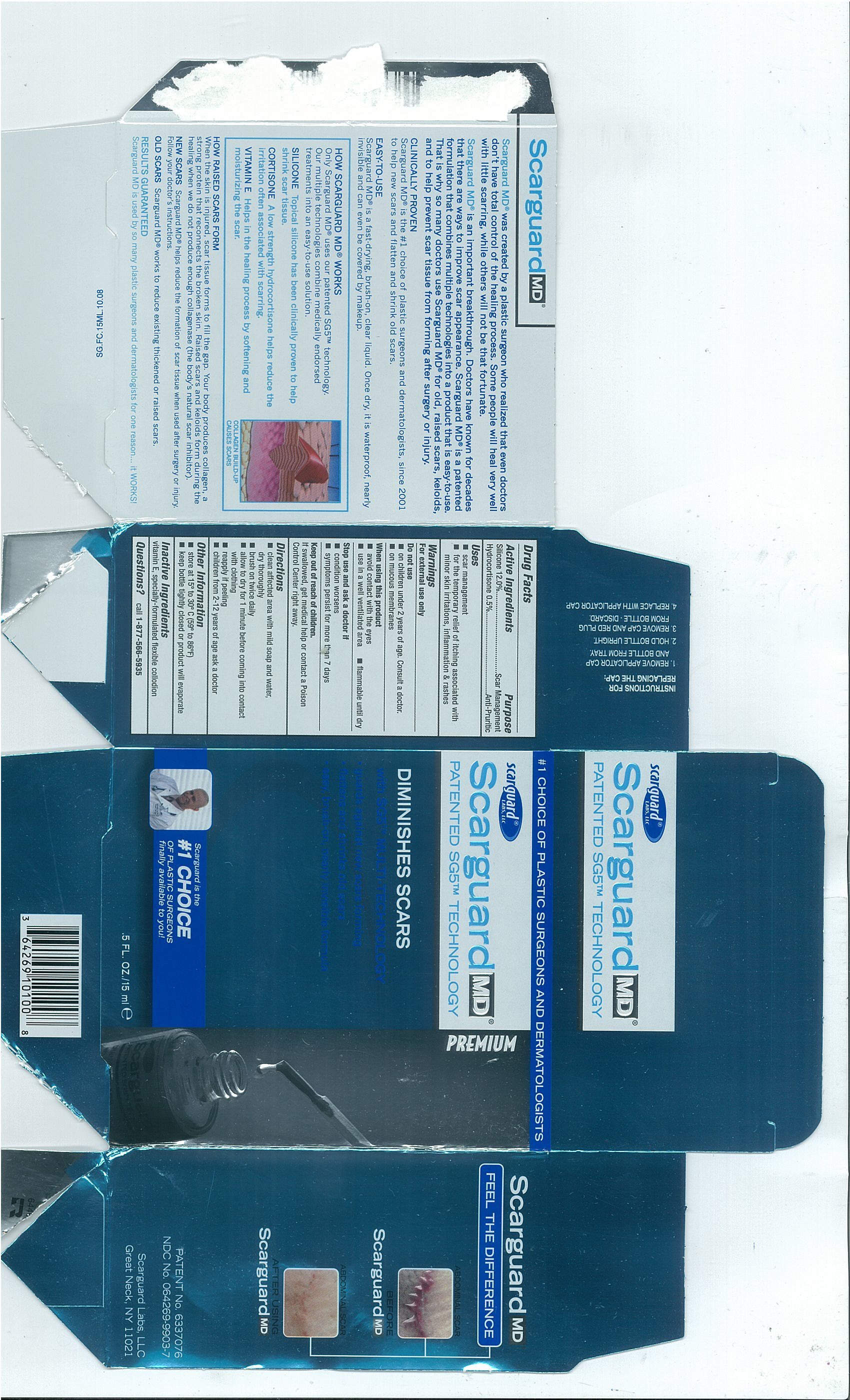
Active Ingredients
- Silicone 12.0%
- Hydrocortisone 0.5%
PurposeScar Management
Anti-Pruritic
Anti-Pruritic
Uses
- scar management
- for the temporary relief of itching associated with minor skin irritations, inflammation and rashes
Warnings
For external use only
Do Not Use
- on children under 2 years of age. Consult a doctor.
- on mucous membranes
When using this product
- avoid contact with the eyes
- use in a well ventilated area
- flammable until dry
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days
Keep out of reach of childrenIf swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean affected area with mild soap and water, dry thoroughly
- brush on twice daily
- allow to dry for 1 minute before coming into contact with clothing
- reapply if peeling
- children from 2-12 years of age ask a doctor
Other Information
- store at 15º to 30º C (59º to 86ºF)
- Keep bottle tightly closed or product will evaporate
Inactive Ingredientsvitamin E, specially-formulated flexible collodion
Questions?call
1-877-566-5935
1-877-566-5935
15 mL carton
| SCARGUARD MD
hydrocortisone silicone liquid |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Scarguard Labs, LLC (842204575) |
| Registrant - Scarguard Labs, LLC (842204575) |
Document Id: 617c546b-3088-405c-bc14-d1759573976e
Set id: eef21e54-c5fc-48ab-a514-97ba805ffea8
Version: 2
Scarguard Labs, LLC
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
