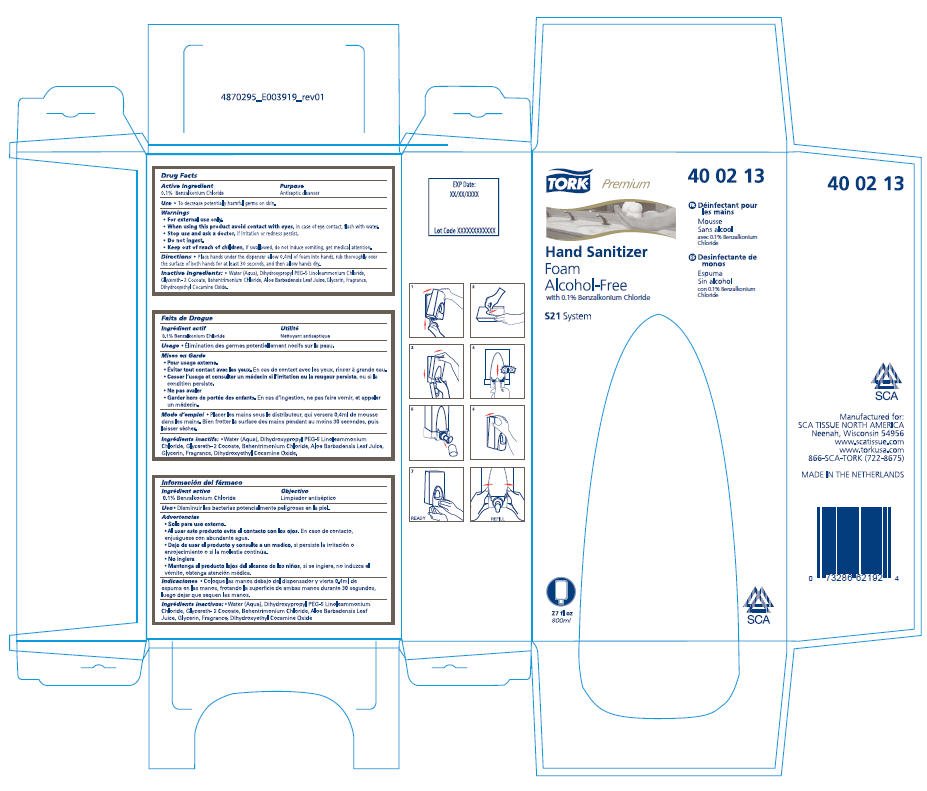
Tork Premium Hand Sanitizer Foam Alcohol-Free
Dosage form: liquid
Ingredients: Benzalkonium Chloride 0.1g in 100mL
Labeler: SCA Tissue North America
NDC code: 49351-016
Medically reviewed by Drugs.com. Last updated on Mar 19, 2025.
Hand Sanitizer
Foam
Alcohol-Free
Drug Facts
0.1% Benzalkonium Chloride
Antiseptic cleanser
- To decrease potentially harmful germs on skin.
- For external use only.
- When using this product avoid contact with eyes, in case of eye contact, flush with water.
- Stop use and ask a doctor, if irritation or redness persist.
- Do not ingest.
- Keep out of reach of children, if swallowed, do not induce vomiting, get medical attention.
- Place hands under the dispenser allow 0.4ml of foam into hands, rub thoroughly over the surface of both hands for at least 30 seconds, and then allow hands dry.
- Water (Aqua), Dihydroxypropyl PEG-5 Linoleammonium Chloride, Glycereth- 2 Cocoate, Behentrimonium Chloride, Aloe Barbadensis Leaf Juice, Glycerin, Fragrance, Dihydroxyethyl Cocamine Oxide.
Manufactured for:
SCA TISSUE NORTH AMERICA
Neenah, Wisconsin 54956
www.scatissue.com
www.torkusa.com
866-SCA-TORK (722-8675)
TORK®
Premium
40 02 13
Hand Sanitizer
Foam
Alcohol-Free
with 0.1% Benzalkonium Chloride
S21 System
FR Déinfectant pour
les mains
Mousse
Sans alcool
avec 0.1% Benzalkonium
Chloride
ES Desinfectante de
monos
Espuma
Sin alcohol
con 0.1% Benzalkonium
Chloride
27 fl oz
800ml
SCA
| TORK PREMIUM HAND SANITIZER FOAM ALCOHOL-FREE
benzalkonium chloride liquid |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
| Labeler - SCA Tissue North America (005694349) |
| Registrant - Rubbermaid Commercial Products LLC (049924368) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.