The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Cepacol Sore Throat Maximum Numbing Cherry
Dosage form: lozenge
Ingredients: BENZOCAINE 15mg, MENTHOL 3.6mg
Labeler: Combe Incorporated
NDC code: 11509-0011
Cēpacol® Sore Throat Maximum Numbing Cherry Lozenges
Benzocaine 15.0 mg
Oral anesthetic
Menthol 3.6 mg
Oral analgesic
temporarily relieves occasional:
- sore throat pain
- sore mouth
- minor mouth irritation
- pain associated with canker sores
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other ‘caine’ anesthetics.
Sore Throat Warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a doctor promptly. Do not use for more than 2 days or give to children under 5 years of age.
sore mouth symptoms do not improve in 7 days, or if irritation, pain or redness persists or worsens.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
Do not exceed recommended dosage.
- adults and children 5 years or older: allow lozenge to dissolve slowly in the mouth; may be repeated every 2 hours as needed or as directed by a doctor or dentist.
- children under 5 years of age: do not use.
- store at room temperature (68-77°F) (20-25°C)
- protect contents from humidity
cetylpyridinium chloride (Ceepryn®), flavor, glucose, potassium acesulfame, propylene glycol, red 33, red 40, sodium bicarbonate, sucrose
Cēpacol® Sore Throat Maximum Numbing Sugar Free Cherry Lozenges
Benzocaine 15.0 mg
Oral anesthetic
Menthol 4.0 mg
Oral analgesic
temporarily relieves occasional:
- sore throat pain
- sore mouth
- minor mouth irritation
- pain associated with canker sores
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other ‘caine’ anesthetics.
Sore Throat Warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a doctor promptly. Do not use for more than 2 days or give to children under 5 years of age.
sore mouth symptoms do not improve in 7 days, or if irritation, pain or redness persists or worsens.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
Do not exceed recommended dosage.
- adults and children 5 years or older: allow lozenge to dissolve slowly in the mouth; may be repeated every 2 hours as needed or as directed by a doctor or dentist.
- children under 5 years of age: do not use.
- store at room temperature (68-77°F) (20-25°C)
- protect contents from humidity
cetylpyridinium chloride (Ceepryn®), flavor, isomalt, maltitol, potassium acesulfame, propylene glycol, red 33, red 40, sodium bicarbonate
Cēpacol® Sore Throat Maximum Numbing Citrus Lozenges
Benzocaine 15.0 mg
Oral anesthetic
Menthol 2.1 mg
Oral analgesic
temporarily relieves occasional:
- sore throat pain
- sore mouth
- minor mouth irritation
- pain associated with canker sores
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other ‘caine’ anesthetics.
Sore Throat Warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a doctor promptly. Do not use for more than 2 days or give to children under 5 years of age.
sore mouth symptoms do not improve in 7 days, or if irritation, pain or redness persists or worsens.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
Do not exceed recommended dosage.
- adults and children 5 years or older: allow lozenge to dissolve slowly in the mouth; may be repeated every 2 hours as needed or as directed by a doctor or dentist.
- children under 5 years of age: do not use.
- store at room temperature (68-77°F) (20-25°C)
- protect contents from humidity
cetylpyridinium chloride (Ceepryn®), flavor, isomalt, maltitol syrup, propylene glycol, sodium bicarbonate, sucralose, yellow 6
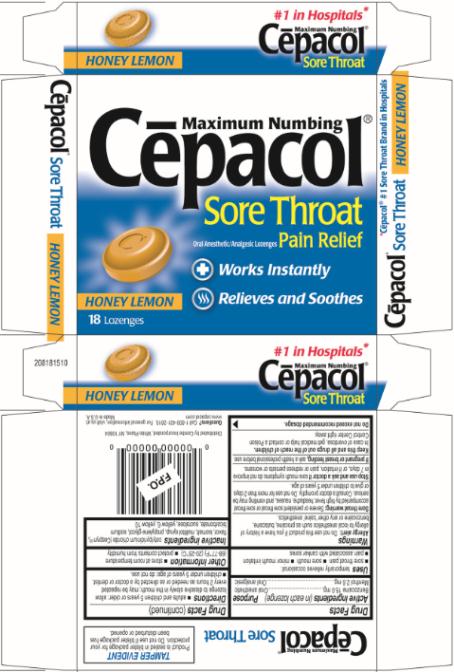
Cēpacol® Sore Throat Maximum Numbing Honey Lemon Lozenges
Benzocaine 15.0 mg
Oral anesthetic
Menthol 2.6 mg
Oral analgesic
temporarily relieves occasional:
- sore throat pain
- sore mouth
- minor mouth irritation
- pain associated with canker sores
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other ‘caine’ anesthetics.
Sore Throat Warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a doctor promptly. Do not use for more than 2 days or give to children under 5 years of age.
sore mouth symptoms do not improve in 7 days, or if irritation, pain or redness persists or worsens.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
Do not exceed recommended dosage.
- adults and children 5 years or older: allow lozenge to dissolve slowly in the mouth; may be repeated every 2 hours as needed or as directed by a doctor or dentist.
- children under 5 years of age: do not use.
- store at room temperature (68-77°F) (20-25°C)
- protect contents from humidity
cetylpyridinium chloride (Ceepryn®), flavor, isomalt, maltitol syrup, propylene glycol, sodium bicarbonate, sucralose, yellow 6, yellow 10.
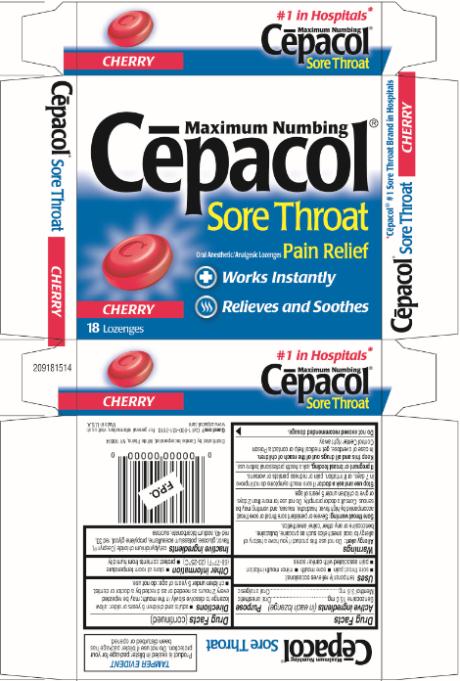
Maximum Numbing
Cēpacol®
Sore Throat
Oral Anesthetic/Analgesic Lozenges Pain Relief
Works Instantly
Relieves and Soothes
CHERRY
18 Lozenges
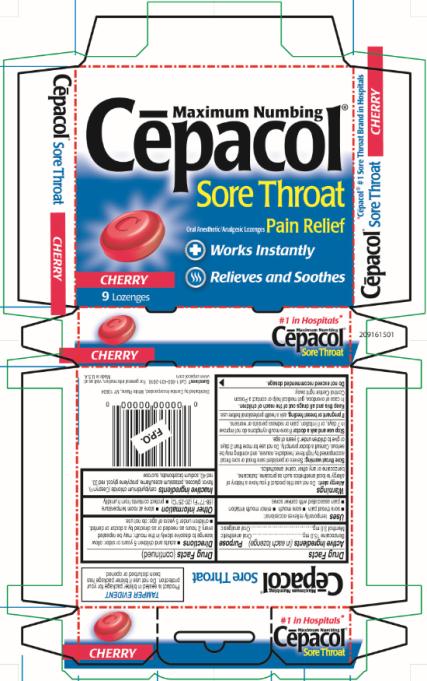
Maximum Numbing
Cēpacol®
Sore Throat
Oral Anesthetic/Analgesic Lozenges Pain Relief
Works Instantly
Relieves and Soothes
CHERRY
9 Lozenges
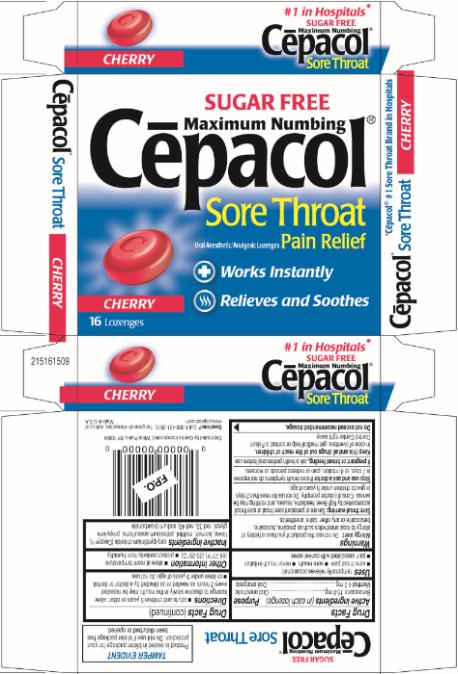
SUGAR FREE
Maximum Numbing
Cēpacol®
Sore Throat
Oral Anesthetic/Analgesic Lozenges Pain Relief
Works Instantly
Relieves and Soothes
CHERRY
16 Lozenges
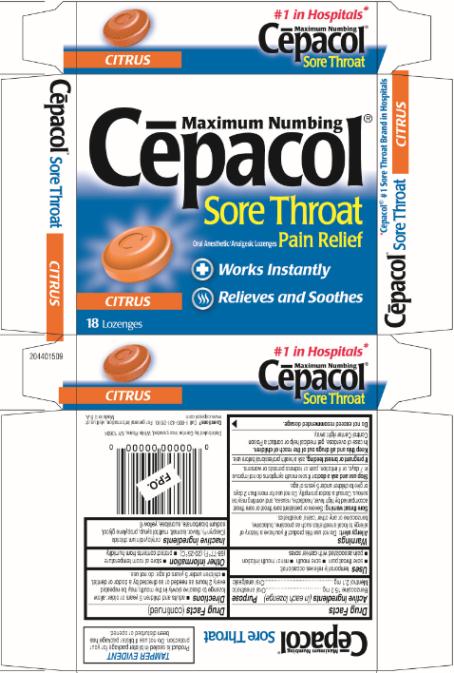
SUGAR FREE
Maximum Numbing
Cēpacol®
Sore Throat
Oral Anesthetic/Analgesic Lozenges Pain Relief
Works Instantly
Relieves and Soothes
CITRUS
18 Lozenges
| CEPACOL SORE THROAT MAXIMUM NUMBING CHERRY
benzocaine and menthol lozenge |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| CEPACOL SORE THROAT MAXIMUM NUMBING SUGAR FREE CHERRY
benzocaine and menthol lozenge |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| CEPACOL SORE THROAT MAXIMUM NUMBING SUGAR FREE CHERRY
benzocaine and menthol lozenge |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| CEPACOL SORE THROAT MAXIMUM NUMBING CITRUS
benzocaine and menthol lozenge |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| CEPACOL SORE THROAT MAXIMUM NUMBING HONEY LEMON
benzocaine and menthol lozenge |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Combe Incorporated (002406502) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| First Boston Pharma LLC | 618613590 | MANUFACTURE | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.