The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
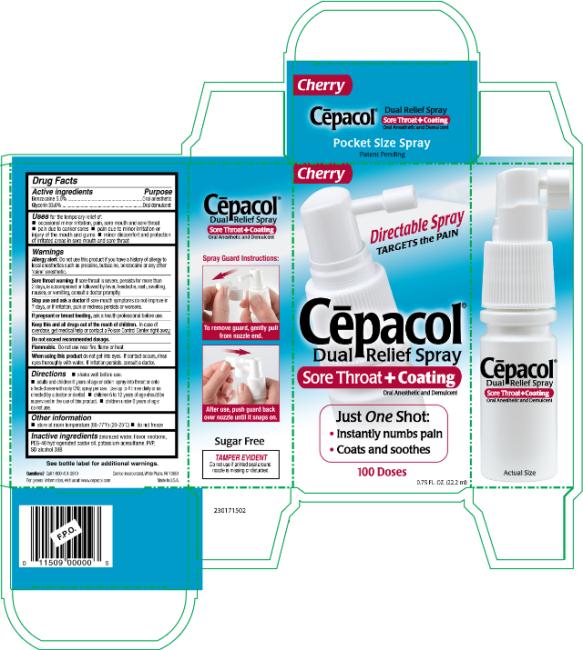
Cepacol Dual Relief
Dosage form: spray
Ingredients: BENZOCAINE 0.0508g in 1mL, GLYCERIN 0.335g in 1mL
Labeler: Combe Incorporated
NDC code: 11509-0054
Cēpacol® Dual Relief Spray
Benzocaine 5.0%
Oral anesthetic
Glycerin 33.0%
Oral demulcent
for the temporary relief of:
- occasional minor irritation, pain, sore mouth and sore throat
- pain due to canker sores
- pain due to minor irritation or injury of the mouth and gums
- minor discomfort and protection of irritated areas in sore mouth and sore throat
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other ‘caine’ anesthetics.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea or vomiting, consult a doctor promptly.
sore mouth symptoms do not improve in 7 days, or if irritation, pain or redness persists or worsens.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
Do not exceed recommended dosage.
Flammable. Do not use near fire, flame or heat.
do not get into eyes. If contact occurs, rinse eyes thoroughly with water. If irritation persists, consult a doctor.
- shake well before use.
- adults and children 6 years of age or older: spray into throat or onto affected area with only ONE spray per use. Use up to 4 times daily or as directed by a doctor or dentist.
- children 6 to 12 years of age should be supervised in the use of this product.
- children under 6 years of age: do not use.
- store at room temperature (68-77°F) (20-25°C)
- do not freeze
deionized water, flavor, neotame, PEG-40 hydrogenated castor oil, potassium acesulfame, PVP, SD alcohol 38B
| CEPACOL DUAL RELIEF
benzocaine and glycerin spray |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Combe Incorporated (002406502) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.