National Drug Codes Explained
What is a National Drug Code (NDC)?
The NDC, or National Drug Code, is a unique 10-digit or 11-digit, 3-segment number, and a universal product identifier for human drugs in the United States. The code is present on all prescription, over-the-counter and insulin products and labels in the U.S. The NDC serves as the FDA’s identifier for drugs.
What are the parts of an NDC?
The 3 segments of the NDC identify the labeler, the product, and the commercial package size.
- Labeler code: The first set of numbers in the NDC identifies the labeler, such as the drug manufacturer, repackager, or distributer.
- Product code: The second set of numbers is the product code, which identifies the specific strength, dosage form (i.e, capsule, tablet, liquid) and formulation of a drug for a specific labeler.
- Package code: The third set of numbers is the package code, which identifies package sizes and types.
The labeler code is assigned by the U.S. Food and Drug Administration (FDA), while the product and package code are assigned by the labeler. For billing or other purposes, such as with the Centers for Medicare & Medicaid Services (CMS), an NDC may also be arranged in an 11-digit format with leading zeros, if needed.
The NDC Directory contains information on all finished and unfinished prescription medications, over-the-counter (OTC) medications and compounded drug products in the U.S. FDA publishes the listed 10-digit NDC numbers and the information submitted as part of the listing information in the NDC Directory which is updated daily.
As of June 1, 2011, only drugs for which electronic listings (Structured Product Labeling or "SPL") have been submitted to FDA are included in the NDC Directory. Animal drugs, blood products, or human drugs, among others, that are not in final marketed electronic form are not included in the NDC directory.
How is the NDC formatted?
The 10-digit NDC will be in one of the following configurations: 4-4-2, 5-3-2, or 5-4-1, meaning that there are 4 or 5 digits for the labeler code, 4 or 3 digits for the product code and 2 or 1 digit(s) for the package code.
Example NDC
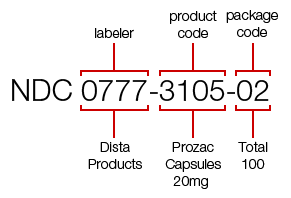
For example, the NDC for a 100-count bottle of Prozac 20 mg is 0777-3105-02.
The first segment identifies the labeler (the labeler code "0777" is for Dista Products Co., the labeler of Prozac).
The second segment, the product code, identifies the strength, dosage form (i.e, capsule, tablet, liquid) and drug formulation for a specific labeler ("3105" identifies that this dosage form is a capsule).
The third segment is the package code, and it identifies package sizes and types. The package code "02" for this bottle of Prozac identifies that 100 capsules are in the bottle.
Where can I find an NDC number for a drug?
- You can easily use the free Drugs.com Pill Identification Wizard to identify medications by NDC number. You can also access NDC numbers via the Drugs.com Medication Guide App or Pill Identiifer app.
- The FDA also maintains a searchable database of NDC codes on their website. NDC numbers can also be found in the drug product labeling (for example, the package insert) as well as on the package itself.
- If you have questions about an NDC for a particular product, check with your pharmacist.
Why are some drug products not in the NDC Directory?
According to the FDA, reasons why a drug product may not appear in the NDC Directory, include:
- The product may not be a prescription or OTC drug, or an insulin product.
- The firm has notified the FDA that the product is no longer commercially available and marketed.
- The manufacturer has not provided a complete listing of the drug product in electronic format.
The FDA requires that drug establishment registration and drug listing information be submitted electronically unless a waiver is granted.
Outsourcing facilities (a type of drug compounding facility) may, but are not required to, assign NDCs to their finished compounded human drug products. Instead, they provide FDA with a list of compounded drugs twice per year. Compounded drugs are medications that are specially formulated by a pharmacist to meet the needs of a patient, usually from a doctor's prescription.
Why do some NDC numbers have 11 digits?
For certain purposes, including the proper billing of drug products, an 11-digit NDC may be required. The Centers for Medicare & Medicaid Services (CMS) and other government entities require an NDC as part of their billing claim form. Some government agencies, including HIPAA, may require the NDC in an 11-digit format (a 5-4-2 format) with leading zeros. Increasingly, private payors are requiring the 11-digit code, but rules can vary greatly.
- The CMS NDC identifier is formatted as 11 digits with no spaces, hyphens or other characters.
- If the NDC Package code is less than 11 digits (for example, a 4-4-2 structure) the code must be padded with leading zeros.
- The leading zero is added to the needed section to create a 5-4-2 configuration.
NDC numbers have also appeared with an asterisk in either a product code or a package code. The asterisk acts as a placeholder and indicates the configuration of the NDC.
Per the FDA, because of a conflict with the HIPAA standard of an 11-digit NDC, many programs will pad the product code or package code segments of the NDC with a leading zero instead of an asterisk. However, the FDA states asterisks are no longer used or included within the product file data elements to indicate certain configurations of the NDC.
Since a zero can be a valid digit in the NDC, this can lead to confusion when trying to return the 11-digit NDC back to its 10-digit FDA standard. For example, as noted by the FDA, 12345-0678-09 (11 digits) could be 12345-678-09 or 12345-0678-9 depending on the firm's configuration.
How do you convert a 10-digit NDC to an 11-digit NDC?
Increasingly, payors are requiring an 11-digit NDC code for billing purposes. Therefore, proper billing may require a specially-placed zero to create a 5-4-2 format depending upon the drug product’s 10-digit NDC.
10-Digit to 11-Digit NDC Conversion Example
| 10-digit format (on package) | 10-digit format (segment format) | Converted 11-digit format (with zero added) | 11-digit format example (for billing purposes) |
|---|---|---|---|
| Table 1: Adapted from Maryland Dept. of Health (www.maryland.gov); FDA National Drug Code Directory | |||
| 9999-9999-99 | 4-4-2 | 09999-9999-99 | 5-4-2 |
| 99999-999-99 | 5-3-2 | 99999-0999-99 | 5-4-2 |
| 99999-9999-9 | 5-4-1 | 99999-9999-09 | 5-4-2 |
How are NDC numbers used for billing purposes?
When submitting a claim for reimbursement, it is always best to check with the payor(s) to determine the specifics for NDC coding and reimbursement, as rules vary widely.
According to the American Academy of Pediatrics (AAP), many payors like Blue Cross and Blue Shield, Tricare, and state Medicaid plans have guidance on how they want NDC codes to be used. In addition, some Medicaid plans exclude the use of NDC codes for vaccines.
When will 10-digit NDC numbers run out?
The FDA states that the 5-digit format provides 90,000 potential combinations but expects to run out of labeler codes by roughly 2033 or even sooner. The COVID-19 pandemic greatly increased the rate at which NDC codes were issued.
Are there 12-digit NCD numbers?
The FDA has proposed a rule for a single, universal 12-digit NDC with one uniform format.
On July 22, 2022 the FDA announced the availability of a proposed rule "Revising the National Drug Code Format and Drug Label Barcode Requirements" (Docket No. FDA-2021-N-1351). The goal is to minimize the impact of FDA running out of ten-digit national drug codes (NDCs) by adopting a single, uniform 12-digit format for FDA-assigned NDCs. FDA is proposing to change the NDC to 12 digits in length with 3 distinct segments and one uniform format.
Under the proposed FDA rule, the NDC would remain a three-segment numerical code consisting of the labeler code (6 digits), the product code (4 digits), and the package code (2 digits) in a single, universal 6-4-2 format (12 digits total). If this rule is finalized, the proposed standardized format would facilitate the adoption of a single NDC format by all stakeholders instead of needing to convert NDCs for payor submission or other uses.
Sources
- Prozac (prescribing information). FDA. Accessed March 20, 2025 at https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/018936%20s112lbl.pdf
- The Anatomy of a National Drug Code. Knowledge Center. Aug 31, 2020. LexisNexis ReedTech. Accessed March 20, 2025 at https://www.reedtech.com/knowledge-center/the-anatomy-of-a-national-drug-code-ndc/
- Proposed Rule on Revising the National Drug Code Format. The U.S. Food and Drug Administration (FDA). Accessed March 20, 2025 at https://www.fda.gov/drugs/drug-approvals-and-databases/proposed-rule-revising-national-drug-code-format
-
National Drug Code (NDC) Directory. The U.S. Food and Drug Administration (FDA). Accessed March 20, 2025 at https://www.fda.gov/Drugs/InformationOnDrugs/ucm142438.htm
-
National Drug Code Database Background Information. The U.S. Food and Drug Administration (FDA). Accessed March 20, 2025 at https://www.fda.gov/drugs/development-approval-process-drugs/national-drug-code-database-background-information
- NDC Product File Definitions. The U.S. Food and Drug Administration (FDA). Accessed March 20, 2025 at https://www.fda.gov/Drugs/InformationOnDrugs/ucm254527.htm
- Millonig M. Are You Prepared for a Major Industry Change to the National Drug Code (NDC) Number? Wolters Kluwer. Accessed June 12, 2024 at https://www.wolterskluwercdi.com/blog/major-industry-change-ndc/
- Revising the National Drug Code Format and Drug Label Barcode Requirements. The U.S. Food and Drug Administration (FDA). Accessed March 20, 2025 at https://www.federalregister.gov/documents/2022/07/25/2022-15414/revising-the-national-drug-code-format-and-drug-label-barcode-requirements#
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
