Tandem F US US US US Pill: brown & red, capsule/oblong, 18mm
Generic Name: ferrous fumarate/folic acid/iron polysaccharide
The pill with imprint Tandem F US US US US (Brown & Red, Capsule/Oblong, 18mm) has been identified as Tandem F 162 mg / 1 mg / 115.2 mg and is used for Iron Deficiency Anemia. It belongs to the drug class iron products and is not a controlled substance.
Images for Tandem F US US US US
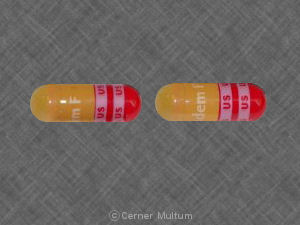
Tandem F
- Generic Name
- ferrous fumarate/folic acid/iron polysaccharide
- Imprint
- Tandem F US US US US
- Strength
- 162 mg / 1 mg / 115.2 mg
- Color
- Brown & Red
- Size
- 18.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- Iron products
- Pregnancy Category
- N - Not classified
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- US Pharmaceutical Corporation
- National Drug Code (NDC)
- 52747-0901 (Discontinued)
See also:
More about Tandem F (ferrous fumarate/folic acid/iron polysaccharide)
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: iron products
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
