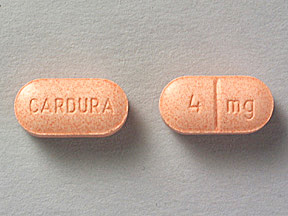
CARDURA 4 mg Pill: orange, oval, 12mm
Generic Name: doxazosin
The pill with imprint CARDURA 4 mg (Orange, Oval, 12mm) has been identified as Cardura 4 mg and is used for Benign Prostatic Hyperplasia, High Blood Pressure, and Raynaud's Syndrome. It belongs to the drug classes alpha blockers, antiadrenergic agents, peripherally acting and is not a controlled substance.
Images for CARDURA 4 mg

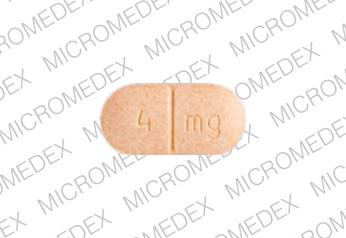
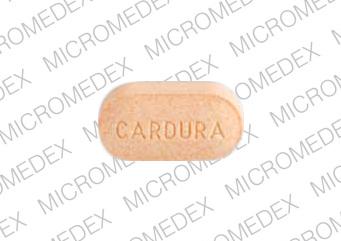
Cardura
- Generic Name
- doxazosin
- Imprint
- CARDURA 4 mg
- Strength
- 4 mg
- Color
- Orange
- Size
- 12.00 mm
- Shape
- Oval
- Availability
- Prescription only
- Drug Class
- Alpha blockers, Antiadrenergic agents, peripherally acting
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Pfizer U.S. Pharmaceuticals Group
- Inactive Ingredients
-
microcrystalline cellulose,
sodium starch glycolate type A potato,
magnesium stearate,
sodium lauryl sulfate,
FD&C Yellow No. 6
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00049-2770 | Pfizer Inc. |
| 59762-2340 (Discontinued) | Viatris |
| 54868-2640 (Discontinued) | Physicians Total Care Inc. (repackager) |
| 66116-0189 | Medvantx Inc. (repackager) |
Related images for "CARDURA 4 mg"
More about Cardura (doxazosin)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (6)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Generic availability
- Drug class: alpha blockers
- Breastfeeding
- En español
Patient resources
Professional resources
Other formulations
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.