Razadyne Dosage
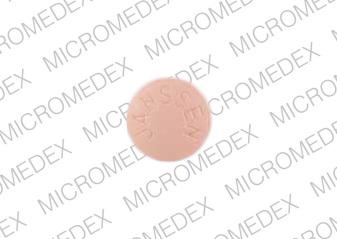
Generic name: galantamine hydrobromide 8mg
Dosage form: capsules, tablets and oral solution
Drug class: Cholinesterase inhibitors
Medically reviewed by Drugs.com. Last updated on Oct 1, 2024.
Recommended Dosage and Administration
Administer RAZADYNE ER extended-release capsules once daily in the morning, preferably with food. Ensure adequate fluid intake during treatment.
The recommended starting dosage of RAZADYNE ER is 8 mg/day. Increase to the initial maintenance dosage of 16 mg/day after a minimum of 4 weeks. A further increase to 24 mg/day may be attempted after a minimum of 4 weeks at 16 mg/day. Increase dosage based upon assessment of clinical benefit and tolerability of the previous dosage.
The dosage of RAZADYNE ER shown to be effective in a controlled clinical trial is 16–24 mg/day.
Dosage in Patients with Hepatic Impairment
In patients with moderate hepatic impairment (Child-Pugh score of 7–9), the dosage should generally not exceed 16 mg/day. The use of RAZADYNE ER in patients with severe hepatic impairment (Child-Pugh score of 10–15) is not recommended.
Dosage in Patients with Renal Impairment
In patients with creatinine clearance of 9 to 59 mL/min, the dosage should generally not exceed 16 mg/day. In patients with creatinine clearance less than 9 mL/min, the use of RAZADYNE ER is not recommended.
Treatment Interruption
If therapy has been interrupted for more than three days, the patient should be restarted at the lowest dosage and the dosage escalated to the current dose.
The abrupt withdrawal of RAZADYNE ER in those patients who had been receiving dosages in the effective range was not associated with an increased frequency of adverse events in comparison with those continuing to receive the same dosages of that drug.
Switching to RAZADYNE ER from Galantamine Tablets
Patients currently being treated with galantamine tablets can convert to RAZADYNE ER (extended-release capsules) by taking their last dose of galantamine tablets in the evening and starting RAZADYNE ER once daily treatment the next morning. Converting from galantamine tablets to RAZADYNE ER should occur at the same total daily dosage.
Frequently asked questions
- What drugs can help improve or slow down memory loss?
- Is Razadyne (galantamine) used to treat Alzheimer’s?
More about Razadyne (galantamine)
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- During pregnancy
- Drug class: cholinesterase inhibitors
Patient resources
Other brands
Professional resources
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.