Lymphoseek Dosage
Generic name: TECHNETIUM TC-99M TILMANOCEPT 250ug
Dosage form: injection
Drug class: Radiologic conjugating agents
Medically reviewed by Drugs.com. Last updated on Sep 8, 2025.
Radiation Safety - Drug Handling
Lymphoseek is a radioactive drug and should be handled with appropriate safety measures to decrease radiation exposure. Use waterproof gloves, effective radiation shielding, and appropriate safety measures when preparing and handling Lymphoseek.
Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
Recommended Dosing
The recommended dose of Lymphoseek is 18.5 MBq (0.5 mCi) as a radioactivity dose and 50 mcg as a mass dose. Administer Lymphoseek at least 15 minutes prior to initiating intraoperative lymphatic mapping and sentinel node biopsy; complete these procedures within 15 hours after Lymphoseek injection.
Route of Administration and Injection Method
The route of administration depends on the tumor location and the planned injection technique and includes: subcutaneous, intradermal, subareolar, or peritumoral injection.
Lymphoseek may be administered to a patient as a single injection or as multiple injections. The recommended total injection volume for each patient (Table 1) is 0.1 mL administered in a single syringe; 0.5 mL administered in a single syringe or in multiple syringes (0.1 mL to 0.25 mL each); or 1 mL administered in multiple syringes (0.2 mL to 0.5 mL each).
The lymphatic system architecture and function may be changed by prior surgery, radiation, edema, inflammation or metastatic disease, and may result in changes to lymph node localization by a radiopharmaceutical or other tracers, including colorimetric agents. Avoid injections into biopsy wound areas that show evidence of edema or inflammation.
In animal studies, locally injected anesthetics have been reported to reduce lymphatic flow. Concomitant administration of local anesthetics with Lymphoseek is not recommended and may impair the lymph nodal mapping.
Drug Preparation
General Considerations
- •
- Lymphoseek (kit for the preparation of technetium Tc 99m tilmanocept injection) contains five vials, each containing 250 mcg of tilmanocept from which 50 mcg is intended for administration to a patient.
- o
- The Lymphoseek kit is packaged either with or without five DILUENT for Lymphoseek vials each containing 4.5 mL of sterile buffered saline with phenol.
- o
- The Lymphoseek kit may also be diluted with pharmacy-available sterile 0.9% sodium chloride injection.
- o
- A diluent is used to dilute Lymphoseek after the radiolabeling procedure. The amount of diluent used varies, depending on the total injection volume and the number of syringes used for each patient.
- •
- The vial components of the Lymphoseek kit are intended solely for use in the preparation of Lymphoseek. Do not administer the unprepared vial components of the kit directly to a patient.
- •
- Follow aseptic procedures during preparation and administration.
Drug Preparation Instructions
Prior to preparation of Lymphoseek, determine the planned injection technique and the number of injections that will be used for a given patient. For each injection prepare a separate syringe. Based on the planned number of injection syringes and the planned total injection volume per patient, determine (from Table 1 below) the Reconstituted Vial Volume of radiolabeled Lymphoseek.
|
Planned Number of |
Total Injection Volume |
Reconstituted Vial Volume of Radiolabeled Lymphoseek |
|
1 syringe x 0.1 mL |
0.1 mL |
0.5 mL |
|
5 syringes x 0.1 mL or 2 syringes x 0.25 mL or 1 syringe x 0.5 mL |
0.5 mL |
2.5 mL |
|
5 syringes x 0.2 mL or 4 syringes x 0.25 mL or 2 syringes x 0.5 mL |
1 mL |
5 mL |
Once the Reconstituted Vial Volume is established, use the following steps to prepare radiolabeled Lymphoseek:
Radiolabeling
a. Inspect the Lymphoseek vial for any damage. Do not use if vial integrity appears compromised. Do not vent the Lymphoseek vial prior to or during radiolabeling.
b. Use Sodium Pertechnetate Tc 99m Injection from a technetium Tc 99m generator within 8 hours of its elution.
c. Using a sterile syringe, aseptically draw approximately 92.5 MBq (2.5 mCi) of Sodium Pertechnetate Tc 99m Injection in either about 0.35 mL volume (for 0.5 mL Reconstituted Vial Volume) or about 0.7 mL volume (for 2.5 mL or 5 mL Reconstituted Vial Volume). Assay the syringe for technetium Tc 99m activity in a dose calibrator.
d. Write the radioactivity amount, the Reconstituted Vial Volume, date and time, expiration time and lot number in the space provided on the radioactive product vial label and affix it to the Lymphoseek vial. Place the vial in a radiation shield and sanitize the septum with alcohol wipe.
e. Aseptically add Sodium Pertechnetate Tc 99m Injection to the Lymphoseek vial. Without withdrawing the needle, remove an equal volume of headspace gas. Do not vent.
f. Remove the needle, gently shake the vial to mix the contents, and then let it stand at room temperature for at least 15 minutes.
Reconstitution
g. Aseptically add the supplied DILUENT for Lymphoseek or pharmacy-available sterile 0.9% sodium chloride injection to the radiolabeled product in the Lymphoseek vial to bring the volume to the Reconstituted Vial Volume of 0.5 mL, 2.5 mL, or 5 mL prior to filling the patient dose in syringe(s). To normalize pressure, withdraw an equal volume of headspace gas.
h. Each Lymphoseek vial, once radiolabeled and reconstituted, would contain sufficient amount to provide doses for up to four patients when prepared according to the instructions.
Quality Control of Radiolabeled Solution
i. Assay the reconstituted vial for total radioactivity using a dose calibrator. Write the technetium Tc 99m activity concentration, total volume, assay time and date, expiration time, and lot number on the shield label supplied with the kit. Affix the label to the shield.
j. Determine the radiochemical purity of the radiolabeled product. Do not use if the radiochemical purity is less than 90%.
k. Withdraw the required volume of the radiolabeled product into the required number of syringes. Assay the syringe(s) in a dose calibrator. Write the radioactivity amount, date and time of assay, volume, and expiration time (this is not to exceed 6 hours from preparation time) on the supplied syringe label and affix it to the syringe(s).
Duration of Use and Storage of Radiolabeled Solution
l. Store the radiolabeled Lymphoseek in radiation shielding at room temperature.
m. Use the radiolabeled Lymphoseek within 6 hours of preparation. Discard the unused radiolabeled Lymphoseek.
Determination of Radiochemical Purity of Radiolabeled Lymphoseek
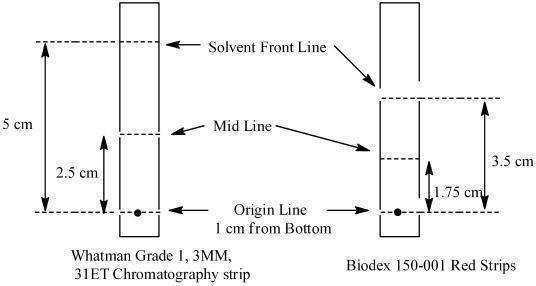
Determine radiochemical purity of the reconstituted radiolabeled Lymphoseek by Instant Thin Layer Chromatography (ITLC) using either Whatman Grade 1, 3MM, 31ET Chr or Biodex 150-001 Red Strips (cellulose chromatography paper) using the following method:
- a.
- Mark the chromatographic strip for origin, mid and solvent front lines with a pencil as shown below:
- b.
- Apply a small drop (3 - 10 microliters) of the reconstituted product at the center of the origin line chromatography strip. Let the product spot dry.
- c.
- Place the strip into a chromatography chamber containing 1 mL of acetone as the developing solvent. Allow the solvent to migrate to the solvent front line (5 cm from the bottom of the Whatman strips and 3.5 cm for the Biodex strip). Remove the strip from the chamber, let it dry and cut it in half. Count each half of the strip with a suitable radioactivity counting apparatus (dose calibrator or multichannel analyzer).
- d.
- Calculate the percent radiochemical purity (% RCP) as follows:
% RCP = Counts (activity) in bottom half x 100
Counts (activity) in bottom half + Counts (activity) in top half
- e.
- Do not use the reconstituted Lymphoseek if the radiochemical purity is less than 90%.
Lymphatic Mapping and Sentinel Lymph Node Biopsy Following Injection of Lymphoseek
- •
- Lymphoscintigraphy may be used to assist in planning the lymph node mapping procedures. In clinical studies, preoperative scintigraphic imaging was performed using planar imaging techniques and/or SPECT/CT to establish a map of nodal basins and to facilitate intraoperative identification of lymph nodes. Imaging was performed as early as immediately after injection and up to 21 hours.
- •
- Use a handheld gamma counter to identify nodes that concentrated the injected radioactivity.
- •
- For intraoperative lymphatic mapping, first measure the background radioactivity counts from tissue at least 20 centimeters distal to the injection site. The three sigma threshold (background radioactivity counts plus three times the square root of the mean background count) may be used as an estimate of the threshold for positive localization of Lymphoseek, as exemplified in Table 2.
| a Average of three 2-second counts or one 10-second count | |
|
Background Counta (cpm) |
Threshold Value (cpm) |
|
5 |
12 |
|
10 |
20 |
|
15 |
27 |
|
20 |
34 |
|
25 |
40 |
|
30 |
47 |
|
35 |
53 |
|
40 |
59 |
- •
- Lymphoseek is intended to supplement palpation, visual inspection, and other procedures important to lymph node mapping and sentinel node biopsy. Intraoperative lymphatic mapping and sentinel node biopsy using gamma detection of Lymphoseek within lymph nodes should be initiated no sooner than 15 minutes following injection. In clinical studies of breast cancer and melanoma, patients also received a concomitant blue dye tracer for comparative detection of lymph nodes. While most lymph nodes were detected with Lymphoseek, some were detected only with the blue dye tracer or only with palpation.
Radiation Dosimetry
- •
- The radiation doses to organs and tissues of an adult patient weighing 70 kg given 18.5 MBq (0.5 mCi) of Lymphoseek are shown in Table 3. For pediatric patients, effective dose equivalent ranged from 355 microSv to 1,232 microSv.
| a Calculated from data of 18 patients with breast cancer who received four peritumoral injections of 4 mcg, 20 mcg, and 100 mcg doses of Lymphoseek. b Calculated from data of 18 patients with melanoma who received four intradermal injections of 20 mcg, 100 mcg, and 200 mcg doses of Lymphoseek. Due to the differences in injection sites among patients with melanoma, the injection site was assumed to be the breast for the purposes of this calculation, as it represents the nearest anatomical construct for the skin from the anatomical sites appropriately included in the estimates. |
||
|
Target Organ |
Breast Cancera |
Melanomab |
|
brain |
0.003 (0.0003) |
0.0927 (0.0093) |
|
breast (injection site) |
1.659 (0.1659) |
0.7903 (0.079) |
|
gall bladder wall |
0.0349 (0.0035) |
0.0712 (0.0071) |
|
lower large intestine wall |
0.0123 (0.0012) |
0.057 (0.0057) |
|
small intestine |
0.0101 (0.001) |
0.0594 (0.0059) |
|
stomach |
0.0184 (0.0018) |
0.0562 (0.0056) |
|
upper large intestine wall |
0.0125 (0.0012) |
0.0582 (0.0058) |
|
kidney |
0.1863 (0.0186) |
0.278 (0.0278) |
|
liver |
0.0324 (0.0032) |
0.0929 (0.0093) |
|
lungs |
0.0374 (0.0037) |
0.0599 (0.006) |
|
muscle |
0.0092 (0.0009) |
0.0451 (0.0045) |
|
ovaries |
0.187 (0.0187) |
0.2991 (0.0299) |
|
red marrow |
0.0127 (0.0013) |
0.0507 (0.0051) |
|
bone |
0.0177 (0.0018) |
0.0878 (0.0088) |
|
spleen |
0.0285 (0.0029) |
0.0598 (0.006) |
|
testes |
0.0501 (0.005) |
0.1043 (0.0104) |
|
thymus |
0.1168 (0.0117) |
0.0577 (0.0058) |
|
thyroid |
0.088 (0.0088) |
0.0464 (0.0046) |
|
urinary bladder |
0.0586 (0.0059) |
0.1401 (0.014) |
|
total body |
0.0195 (0.0019) |
0.0547 (0.0055) |
|
Effective Dose Equivalent males females |
microSv 296 330.2 |
microSv 202.4 251.1 |
More about Lymphoseek (tilmanocept)
- Check interactions
- Compare alternatives
- Reviews (1)
- Side effects
- During pregnancy
- FDA approval history
- Drug class: radiologic conjugating agents
- Breastfeeding
Patient resources
Professional resources
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.