Kovaltry Dosage
Generic name: ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 250[iU] in 2.5mL;
Dosage form: injection
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on Nov 21, 2024.
For intravenous use after reconstitution only.
Dose
- •
- Dosage and duration of treatment depend on the severity of the Factor VIII deficiency, the location and extent of bleeding, and the patient’s clinical condition. Careful control of replacement therapy is especially important in cases of major surgery or life-threatening bleeding episodes.
- •
- Each vial label of KOVALTRY states the Factor VIII potency in international units (IU). One IU is defined by the current WHO (World Health Organization) international standard (IS) for Factor VIII concentrate.
- •
- Potency assignment for KOVALTRY is determined using a chromogenic substrate assay. A field study involving 41 clinical laboratories from around the world measured recoveries of KOVALTRY spiked into hemophilic plasma. The results of the field study indicated that the Factor VIII activity of KOVALTRY can be accurately measured in plasma using either a one-stage clotting or chromogenic substrate assay according to routine methods of the testing laboratory.
- •
- The required dose for a desired Factor VIII level expressed as IU/dL (or % of normal) can be estimated using the following formula:
- Required dose (IU) = body weight (kg) x desired Factor VIII rise (% of normal or IU/dL)
x reciprocal of expected/observed recovery (e.g., 0.5 for a recovery of 2 IU/dL per IU/kg) - The expected in vivo peak increase of Factor VIII level expressed as IU/dL (or % of normal) can be estimated using the following formula:
- Estimated increment of Factor VIII (IU/dL or % of normal) = [Total dose (IU)/body weight (kg)]
x 2 (IU/dL per IU/kg) - Examples (assuming patient’s baseline Factor VIII is <1%):
- •
- A peak of 50% [50 IU/dL] is required in a 20 kg child. In this situation, the required dose of KOVALTRY would be 20 kg x 50 IU/dL x 0.5 (for recovery of 2 IU/dL per IU/kg) = 500 IU
- •
- A dose of 2000 IU of KOVALTRY administered to a 50 kg patient should be expected to result in post-infusion Factor VIII increase of 2000 IU / 50 kg (body weight) x 2 IU/dL per IU/kg = 80 IU/dL (80% of normal)
- •
- Adjust dose to the patient’s clinical response. Patients may vary in their pharmacokinetic (e.g., half-life, incremental recovery) and clinical responses to KOVALTRY.
- •
- On-demand Treatment and Control of Bleeding Episodes
A guide for dosing KOVALTRY for the on-demand treatment and control of bleeding episodes is provided in Table 1. The goal of treatment is to maintain a plasma Factor VIII activity level at or above the plasma levels (in % of normal or in IU/dL) outlined in Table 1.
|
Degree of Bleeding |
Factor VIII Level Required |
Frequency of Doses (hours) |
Duration of Therapy (days) |
|
Minor (Early hemarthrosis, minor muscle, oral bleeds) |
20–40 |
Repeat every |
At least 1 day, until bleeding episode as indicated by pain is resolved or healing is achieved |
|
Moderate (More extensive hemarthrosis, muscle bleeding, or hematoma) |
30–60 |
Repeat every |
3 to 4 days or more until pain and acute disability are resolved |
|
Major (intracranial, intra-abdominal or intrathoracic hemorrhages, gastrointestinal bleeding, central nervous system bleeding, bleeding in the retropharyngeal or retroperitoneal spaces, or iliopsoas sheath, life or limb threatening hemorrhage) |
60–100 |
Repeat every |
Until bleeding is resolved |
Perioperative Management of Bleeding
A guide for dosing KOVALTRY during surgery (perioperative management) is provided in Table 2. The goal of treatment is to maintain a plasma Factor VIII activity level at or above the plasma level (in % of normal or in IU/dL) outlined in Table 2. During major surgery, monitoring with appropriate laboratory tests, including serial Factor VIII activity assays, is highly recommended.
|
Type of Surgery |
Factor VIII Level Required |
Frequency of Doses (hours) |
Duration of Therapy (days) |
|
Minor (Such as tooth extraction) |
30–60 |
Repeat every 24 hours |
At least 1 day until healing is achieved |
|
Major (Such as intracranial, intra-abdominal, intrathoracic, or joint replacement surgery) |
80–100 |
Repeat every |
Until adequate wound healing is complete, then continue therapy for at least another 7 days to maintain Factor VIII activity of |
Routine Prophylaxis
- •
- Individualize the patient’s dose based on clinical response.
- •
- Adults and adolescents: 20 to 40 IU of KOVALTRY per kg of body weight two or three times per week.
- •
- Children ≤12 years old: 25 to 50 IU of KOVALTRY per kg body weight twice weekly, three times weekly, or every other day according to individual requirements.
Preparation and Reconstitution
- •
- Reconstitute and administer KOVALTRY with the components provided with each package. If any component of the package is opened or damaged, do not use this component.
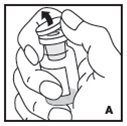
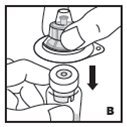
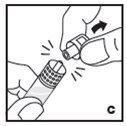
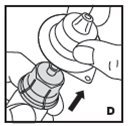
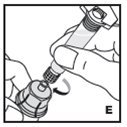
The procedures below are provided as general guidelines for the reconstitution of KOVALTRY using the sterile vial adapter with a 15 micrometer filter and a prefilled diluent syringe, which together serve as an alternative needleless reconstitution system.
Usability Testing of Vial Adapter
Usability testing was conducted with 60 users, including 15 pediatric hemophilia A patients (between 10-17 years of age), 15 adult hemophilia A patients (≥18 years of age), 15 caregivers, and 15 healthcare providers. To mimic real life, the pediatric and adult patients and the caregivers were given minimal training, which included participants performing a supervised reconstitution and later performing a single unaided reconstitution. Healthcare providers were untrained in this study and could learn the procedure from the provided Instructions for Use. All participants were able to successfully and safely use the vial adapter device for reconstitution.
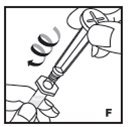
Reconstitution
- •
- Work on a clean surface and wash hands thoroughly using soap and warm water before performing the procedures.
- •
- Reconstitute KOVALTRY with the components provided with each package. If any component of the package is opened or damaged, do not use this component.
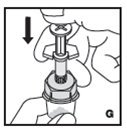
- •
- Filter the reconstituted product to remove potential particulate matter in the solution. Filtering is achieved by using the vial adapter.
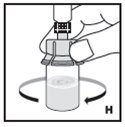
Pooling
If the dose requires more than one vial, reconstitute each vial as described above with the diluent syringe provided. Use a larger plastic syringe (not provided) to combine the content of the vials into the syringe.
Administration
For intravenous use only.
- •
- Inspect reconstituted KOVALTRY visually for particulate matter and discoloration prior to administration. Do not use if you notice any particulate matter or discoloration.
- •
- Administer reconstituted KOVALTRY as soon as possible. If not, store at room temperature for no longer than 3 hours.
- •
- Infuse KOVALTRY intravenously over a period of 1 to 15 minutes. Adapt the rate of administration to the response of each individual patient.
More about Kovaltry (antihemophilic factor)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous coagulation modifiers
- Breastfeeding
- En español
Patient resources
Other brands
Advate, Altuviiio, Eloctate, Esperoct, ... +14 more
Professional resources
- Kovaltry prescribing information
- Antihemophilic Factor (recombinant), PEGylated-aucl (AHFS Monograph)
Other brands
Advate, Altuviiio, Eloctate, Esperoct, ... +12 more
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.