Encelto Dosage
Generic name: REVAKINAGENE TARORETCEL 440000[arb'U]
Dosage form: intravitreal implant
Drug class: Miscellaneous uncategorized agents
Medically reviewed by Drugs.com. Last updated on Mar 21, 2025.
Recommended Dose
For intravitreal implantation only
• ENCELTO is administered by a single surgical intravitreal procedure performed by a qualified ophthalmologist.
• The recommended dose is one ENCELTO implant per affected eye. Each ENCELTO implant contains 200,000 to 440,000 allogeneic retinal pigment epithelial cells expressing recombinant human ciliary neurotrophic factor (rhCNTF) (NTC-201-6A cell line), a neurotrophic factor.
ENCELTO Surgical Placement
The ENCELTO implant insertion is a surgical procedure performed in an operating room under aseptic conditions by a qualified ophthalmologist.
|
Pre-Surgical Preparation
Prepare the surgical field properly. |
|
|
Surgical Steps |
|
|
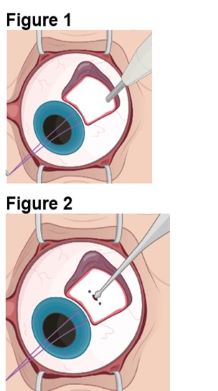
1. Preparing the Surgical Site a. Create a 7x7 mm peritomy of the conjunctiva and Tenon’s capsule at the selected implantation site. b. Place a corneal-limbal traction suture in the selected surgical quadrant (either inferotemporal or inferonasal) (Figure 1). c. Maintain hemostasis of the underlying sclera and conjunctiva (Figure 1). d. Using an MVR and 15-degree blade, create a 3.0 mm full-thickness sclerotomy 3.75 mm posterior and parallel to the limbus (Figure 2). Do not insert ENCELTO outside of the pars plana. e. Confirm: • The incision is full thickness. • There is adequate hemostasis. • There is no spanning uveal tissue. |
|
|
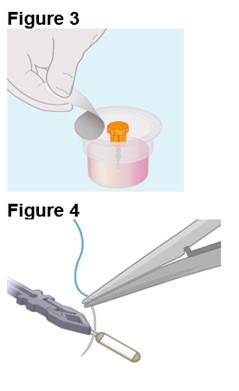
2. Preparing the ENCELTO Implant a. Open the inner container and expose the upper compartment and luer lock cap (Figure 3). b. Unlock the luer lock cap by turning it counterclockwise once. c. Lift the luer lock cap vertically to remove ENCELTO (attached to the gripper). d. Rinse ENCELTO with at least 5 mL of sterile Balanced Saline Solution (BSS). e. Keep ENCELTO moist by applying BSS every 10 minutes until insertion. f. While holding the luer lock cap, pass a double-armed 9-0 polypropylene suture needle through ENCELTO’s fixation loop (Figure 4). |
|
|
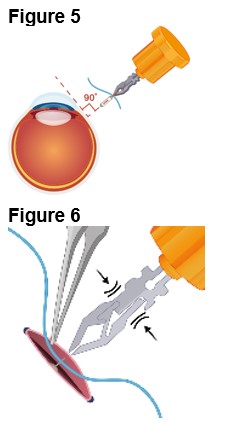
3. Implantation of ENCELTO a. Gently open the sclerotomy incision and insert ENCELTO perpendicularly into the eye (Figure 5). b. Ensure only the fixation loop is exposed. c. Release ENCELTO from the gripper by squeezing the indicated region with forceps or a fine needle holder (Figure 6). |
|
|
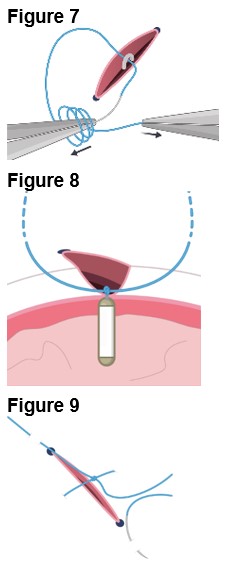
4. Securing the Implant a. Secure ENCELTO by creating a 3-1-1 anchor knot with the polypropylene suture at the apex of the fixation loop (Figure 7). b. Confirm ENCELTO is centered in the incision. c. Pass each suture arm centrally through either side of the wound at 90-99% scleral depth (Figure 8). d. Pull up the suture ends and confirm that the fixation loop is at the proper depth (90-99%). e. Tie down the suture to the sclera with a 3-1-1 knot, ensuring the knot is placed away from the incision. f. If a suture breaks, leave the tail as long as possible and lay it flat. g. Take a 2.0 mm scleral bite at 50-75% depth beyond the sclerotomy on each side (Figure 9). |
|
|
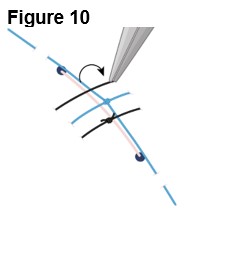
5. Closing the Incision a. Close the scleral incision with 9-0 nylon sutures (Figure 10), ensuring:
b. Pull the polypropylene suture end taut and cut it flush to the sclera. c. Close the conjunctiva and Tenon’s capsule using 6-0 plain gut or chromic suture, or 7-0 Vicryl suture or similar. d. Ensure Tenon’s capsule covers the insertion site and use 3-point fixation and scleral bites. e. Administer sub-conjunctival steroid injection: dexamethasone, 2 mg/0.5 ml (4 mg/ml) or equivalent. If the case is complicated and inflammation is anticipated, a higher dose of dexamethasone (0.5 cc of 10 mg/ml) or equivalent may be used, at the surgeon’s discretion. f. Perform indirect ophthalmoscopy to confirm placement of ENCELTO in the vitreous and that there are no intraocular complications. Failure to perform indirect ophthalmoscopy can lead to unidentified malpositioning of ENCELTO and intraocular complications. |
|
|
Post-Operative Wound Care
|
|
Refer to ENCELTO Instructions for Use for detailed guidance on implantation procedure.
ENCELTO Removal Procedure
Removal of ENCELTO is a surgical procedure performed in an operating room under aseptic conditions by a qualified ophthalmologist. Remove ENCELTO implant, if vitrectomy with a complete gas fill or silicone oil fill is required or if infectious endophthalmitis occurs.
|
Surgical Steps |
|
|
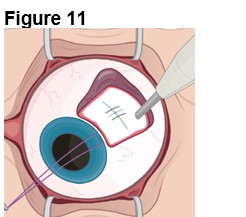
1. Preparing the Surgical Site (Figure 11) a. Create a 7x7 mm peritomy of the conjunctiva and Tenon’s capsule to expose the insertion site. b. Place a corneal-limbal traction suture in the quadrant where ENCELTO is located. c. Maintain hemostasis of the sclera and surrounding conjunctiva. |
|
|
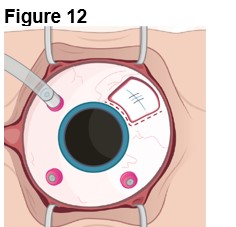
2. Establishing Infusion & Vitrectomy (Figure 12) a. Place an infusion cannula in the inferior quadrant (opposite ENCELTO). b. Confirm the infusion line is positioned within the vitreous cavity before opening the infusion. c. Insert two superior cannulas following normal pars plana vitrectomy protocol. d. Perform a thorough vitrectomy to remove vitreous surrounding ENCELTO without disrupting the hollow fiber membrane. |
|
|
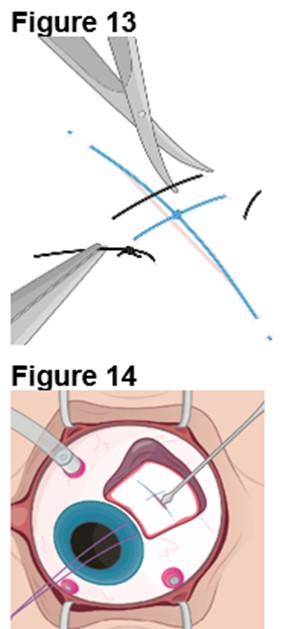
3. Reopening the Sclerotomy a. Locate the ENCELTO incision and remove the two nylon sutures while leaving the polypropylene suture intact (Figure 13). b. Using an MVR blade, carefully dissect open the original scleral incision down to the ENCELTO cap at the base of the fixation loop (Figure 14). c. Extend the incision along the entire 3.0 mm length to full thickness. d. Cut the polypropylene anchor suture on the anterior side of the knot. e. Turn off or lower infusion pressure. |
|
|
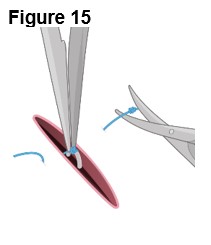
4. Removing ENCELTO (Figure 15) a. Fully open the pars plana sclerotomy and confirm there is no spanning uveal tissue. b. Identify and grasp the fixation loop. c. Cut off the remaining polypropylene knot. d. Remove ENCELTO from the eye. e. Inspect the ENCELTO capsule for any damage or penetration. f. Do not discard or dispose of the ENCELTO implant. Call and report to 1-833-963-9275. The appropriate action will be taken to initiate the return of ENCELTO and possible replacement. |
|
|
5. Closing the Incision a. Remove any prolapsed vitreous. b. Close the sclerotomy with interrupted 7-0 Vicryl sutures for a watertight closure. c. Remove the infusion line and additional cannulas. d. Close the conjunctiva with 6-0 plain gut sutures or equivalent. |
|
|
Post-Operative Wound Care
|
|
Refer to ENCELTO Instructions for Use for detailed guidance on removal procedure.
More about Encelto (revakinagene taroretcel)
- Check interactions
- Compare alternatives
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous uncategorized agents
- En español
Patient resources
Professional resources
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.