7176 9 3 Pill - blue oval, 11mm
Pill with imprint 7176 9 3 is Blue, Oval and has been identified as Sertraline Hydrochloride 50 mg. It is supplied by Teva Pharmaceuticals USA.
Sertraline is used in the treatment of Major Depressive Disorder; Obsessive Compulsive Disorder; Panic Disorder; Depression; Post Traumatic Stress Disorder and belongs to the drug class selective serotonin reuptake inhibitors. Risk cannot be ruled out during pregnancy. Sertraline 50 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for 7176 9 3
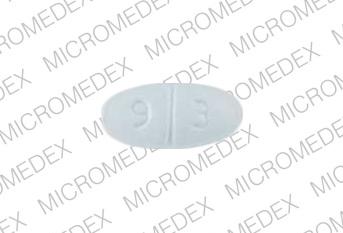
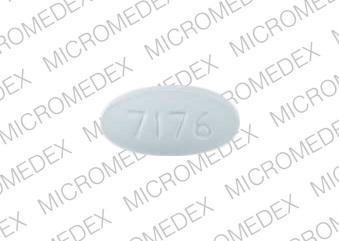
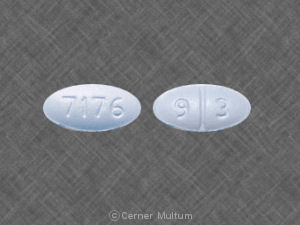
Sertraline Hydrochloride
- Imprint
- 7176 9 3
- Strength
- 50 mg
- Color
- Blue
- Size
- 11.00 mm
- Shape
- Oval
- Availability
- Prescription only
- Drug Class
- Selective serotonin reuptake inhibitors
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Teva Pharmaceuticals USA
- National Drug Code (NDC)
- 00093-7176 (Discontinued)
See also:
Auvelity
Auvelity (dextromethorphan and bupropion) is used to treat major depressive disorder. Includes ...
Lexapro
Lexapro is used to treat anxiety and major depressive disorder. Learn about side effects ...
Vraylar
Vraylar is a once a day antipsychotic medication used to treat mental health or mood disorders ...
Zoloft
Zoloft is an antidepressant used to treat major depression, obsessive-compulsive disorder, panic ...
Seroquel
Seroquel is used to treat the symptoms of schizophrenia, bipolar disorder and major depressive ...
Prozac
Prozac (fluoxetine) is an SSRI antidepressant used to treat depression, OCD, panic disorder ...
Cymbalta
Cymbalta (duloxetine) is used to treat major depressive disorder, general anxiety disorder and ...
Wellbutrin
Wellbutrin (bupropion) is used to treat major depressive disorder and seasonal affective disorder ...
Duloxetine
Duloxetine is a selective serotonin and norepinephrine reuptake inhibitor antidepressant used to ...
More about sertraline
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (4,582)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: selective serotonin reuptake inhibitors
- Breastfeeding
- En español
Patient resources
Other brands
Professional resources
- Sertraline monograph
- Sertraline (FDA)
- Sertraline Capsule (FDA)
- Sertraline ER Tablets (FDA)
- Sertraline Oral Concentrate (FDA)
- Sertraline Oral Solution (FDA)
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
