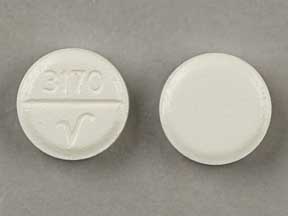
3170 V Pill - white round, 8mm
Pill with imprint 3170 V is White, Round and has been identified as Furosemide 40 mg. It is supplied by Qualitest Pharmaceuticals Inc.
Furosemide is used in the treatment of Edema; Heart Failure; Ascites; High Blood Pressure; Chronic Kidney Disease and belongs to the drug class loop diuretics. Risk cannot be ruled out during pregnancy. Furosemide 40 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for 3170 V
Furosemide
- Imprint
- 3170 V
- Strength
- 40 mg
- Color
- White
- Size
- 8.00 mm
- Shape
- Round
- Availability
- Prescription only
- Drug Class
- Loop diuretics
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Qualitest Pharmaceuticals Inc.
- Inactive Ingredients
-
corn starch,
lactose monohydrate,
magnesium stearate,
magnesium silicate
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00603-3740 (Discontinued) | Qualitest Pharmaceuticals |
| 49999-0030 (Discontinued) | Lake Erie Medical and Surgical Supply (repackager) |
| 54569-0574 (Discontinued) | A-S Medication Solutions, LLC (repackager) |
| 55289-0118 (Discontinued) | PDRX Pharmaceuticals Inc. (repackager) |
See also:
Lasix
Lasix is a loop diuretic used to treat fluid retention from heart, liver, or kidney conditions, and ...
Botox
Botox is used for cosmetic purposes and to treat overactive bladder symptoms, urinary incontinence ...
Aldactone
Aldactone (spironolactone) is used to diagnose or treat a condition in which you have too much ...
Triamterene
Triamterene (Dyrenium) is used to treat fluid retention in people with congestive heart failure ...
Acetazolamide
Acetazolamide is used for edema, epilepsy, glaucoma, hydrocephalus, hypokalemic periodic paralysis ...
Bumetanide
Bumetanide is used for ascites, autism, edema, pulmonary edema
More about furosemide
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (121)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: loop diuretics
- Breastfeeding
- En español
Patient resources
Other brands
Professional resources
- Furosemide monograph
- Furosemide (FDA)
- Furosemide Injection (FDA)
- Furosemide Oral Solution (FDA)
- Furosemide Tablets (FDA)
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
