The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
ViraCHEK CV
This treatment applies to the following species:FELINE INFECTIOUS PERITONITIS VIRUS ANTIBODY TEST KIT
For the detection of Feline Corona Virus (FeCV) antibodies in feline serum or plasma.
GENERAL INFORMATION AND INTENDED USES
ViraCHEK® CV is highly specific, sensitive and simple to perform for the detection of Feline Corona Virus (FeCV) antibodies. Test results can be obtained in 30 minutes. The diagnostic kit contains a positive control and a negative control which should be included each time the assay is performed. Visual comparison of the color of samples to the negative control will allow accurate detection of the presence of FeCV antibody in the sample.
The plastic wells are coated with purified coronavirus antigen. Protein A derived from Staphylococcus aureus is conjugated to HRP. The serum or plasma sample is incubated in the coated wells followed by incubation with the Protein A conjugate. Antibodies to FeCV, if present in the feline sample, are bound to the well and in turn bind the Protein A conjugate. The free enzyme-linked Protein A is washed away and a chromogenic substrate is added. The development of a distinctly blue color indicates the presence of antibody to FeCV. In the absence of FeCV antibody, no color change will be observed.
KIT COMPOSITION AND CONSERVATION
Contains materials sufficient to test 32 - 94 samples.
|
ITEM |
REAGENT NATURE |
VOLUME |
RECONSTITUTION AND CONSERVATION |
|
A |
FeCV Antigen Coated Wells |
2 sets of 4x12 wells |
Ready to use. CDV wells have a white rim. |
|
CONTROL + |
Positive Control |
1.5 mL |
Ready to use. Red Cap. |
|
B |
Negative Control/Sample Diluent; preserved with Phenol and Gentamicin sulfate |
5.0 mL |
Ready to use. Gray Cap. |
|
C |
Conjugate |
5.0 mL |
Ready to use. Blue Cap. |
|
D |
Chromogen |
7.5 mL |
Ready to use. Green Cap. |
|
E |
Substrate Buffer; preserved with Sodium Benzoate |
7.5 mL |
Ready to use. White Cap. |
|
F |
10X Wash Concentrate; preserved with Gentamicin sulfate. |
100 mL |
Dilute to 1X in deionized or distilled water. Orange Cap. Diluted Wash Solution may be stored at 2 - 7 °C. |
|
|
Disposable Sample Loops |
2 bags x 48 loops |
Ready to use. |
|
|
Well Holder |
1 |
Ready to use. |
Note: All reagents provided in the kit should be stored at 2 - 7 °C. Reagents should not be frozen.
REAGENTS REQUIRED TO PERFORM 24 TESTS
a) 4 x 12 FeCV Antigen Coated Strips (2 sets)
b) 1.5 mL Positive Control
c) 5.0 mL Negative Control/Sample Diluent
d) 5.0 mL Conjugate
e) 7.5 mL Chromogen
f) 7.5 mL Substrate Buffer
g) 100 mL 10X Wash
h) 1 Well holder
EQUIPMENT AND MATERIALS REQUIRED BUT NOT PROVIDED
a) Deionized or distilled water
b) 2 Squirt bottles
c) Timer
WARNINGS TO THE USERS OF REAGENTS AND ANTIGEN COATED MICROPLATES
● Handle all reagents and samples as biohazardous material. It is recommended to dispose reagents and contaminated material according to the applicable regulations.
● Wear suitable protective clothing.
● Irritating to eyes and skin. Keep all reagents away from eyes and skin. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
● Take care not to contaminate any test reagents with serum or bacterial agents.
● The best results are achieved by following the protocols described below, using good, safe laboratory techniques.
● Do not use this kit or any of its contents after the expiration date.
● Do not intermix components from different serial numbers.
● Use a separate sample loop for each sample.
● Do not expose kit to direct sunlight.
● NEVER PIPETTE BY MOUTH. Harmful if swallowed.
DANGER
Chromogen Substrate: Danger. Causes serious eye irritation. May damage the unborn child. May cause cancer. Harmful to aquatic life with long lasting effects. Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Wear protective gloves/protective clothing/eye protection/face protection. Wash thoroughly after handling. Avoid release to the environment. If exposed or concerned: Get medical advice/attention. If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. Store locked up. Dispose of contents/container in accordance with local/regional/national/international regulations.
SAMPLE COLLECTION AND STORAGE
● Follow proper sample collection procedures.
● Harvest serum and store properly (up to seven days at 4 °C; -20 °C for longer).
● Test only good quality serum or plasma (i.e. avoid bacterial contamination, heavy hemolysis or clotted fat). When in doubt, obtain a better quality sample.
● Allow all reagents to come to 21 - 24 °C before starting.
PREPARATION OF WASH SOLUTION
Allow 10X wash concentrate to come to ambient temperature. Mix gently by inversion. Dilute wash concentrate 10-fold with distilled or deionized water (1 part concentrate to 9 parts deionized or distilled water) in a squirt bottle. Diluted wash solution may be stored at 2 - 7 °C.
TEST PROCEDURE
|
STEP |
NOTES |
|
|
SET UP AND SAMPLE INCUBATION |
||
|
1) |
Remove and place in well holder one well for Positive Control, one well for Negative Control, and one well for each sample. Leave the wells attached to each other. |
 |
|
2) |
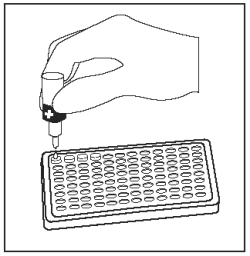
Add 1 drop of Positive Control (Red Cap) into the first well. |
 |
|
3) |
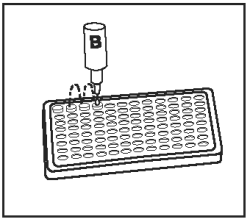
Add 1 drop of Negative Control/Sample Diluent (Bottle B - Gray Cap) into the second well and to each test sample well. |
 |
|
4) |
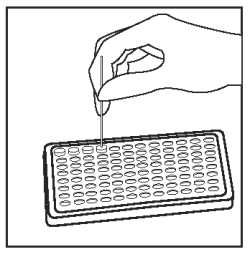
Using a separate sample loop for each sample, add 1 loopful (1 µL) of each sample to appropriate wells. Mix sample in diluent thoroughly by twisting the handle of the loop between the thumb and forefinger. Be careful not to splash from well to well. |
 |
|
5) |
Swirl samples gently to mix. |
|
|
6) |
Incubate for 10 minutes at 21 - 25 °C. |
|
|
7) |
NOTE: If several samples are run simultaneously, only one set of controls is needed. |
|
|
BLOT AND WASH PROCEDURE |
||
|
8) |
Discard the fluid from the wells by inverting and blotting onto a paper towel. Wash wells once by vigorously filling the wells to overflowing with diluted wash solution. |
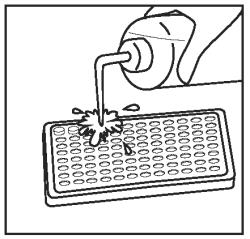 |
|
9) |
Discard the fluid from the wells, and blot after wash. |
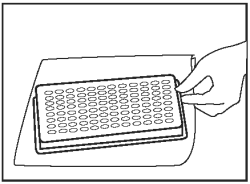 |
|
ADDITION OF CONJUGATE |
||
|
10) |
Add 1 drop of Conjugate (Bottle C - Blue Cap) into each well. Gently tap the holder for 10 - 15 seconds. |
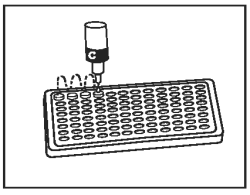 |
|
11) |
Incubate for 10 minutes at 21 - 25 °C. |
|
|
BLOT AND WASH |
||
|
12) |
Discard the fluid from the wells by inverting and blotting onto a paper towel. Wash by vigorously filling the wells to overflowing with diluted wash solution. |
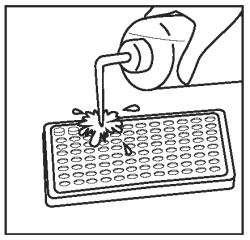 |
|
13) |
Discard the fluid from the wells into an appropriate container and blot after each wash. |
 |
|
14) |
Repeat the wash procedure (steps 12 and 13) three (3) times. |
|
|
15) |
Wash two (2) more times with distilled or deionized water to remove bubbles. |
|
|
16) |
Blot dry on a paper towel. |
|
|
DEVELOP |
||
|
17) |
Place 1 drop of Chromogen (Bottle D - Green Cap) into each well, followed by 1 drop of Substrate Buffer (Bottle E - White Cap). Mix by gently tapping the holder several times. |
 |
|
18) |
Incubate 5 minutes. |
|
|
19) |
After incubating, gently tap holder for 5 seconds and read results immediately. See Interpretation of Results section. |
|
INTERPRETATION OF RESULTS
Controls:
● POSITIVE control should be distinctly blue.
● NEGATIVE control should be completely clear.
Samples:
● POSITIVE samples will be blue. A positive result is indicated by any degree of blue color in the sample well.
● NEGATIVE samples will be clear with no blue color development in the sample well.
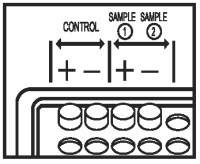
A Negative result (antibody to feline coronavirus is absent) is indicated by no blue color development in the sample well. A Negative test result indicates that the animal is free of the virus or has not yet sero-converted after exposure. Cats may sero-convert in as little as 4 weeks or as long as twelve weeks after becoming infected with Feline Coronavirus. Care must be taken when interpreting positive test results in cats under six months of age as maternal FeCV antibodies may be present.
NOTES
● Use plasma or serum samples only (no whole blood).
● Washing is the most important step. Microwells cannot be overwashed. Underwashing will result in nonspecific color development in the negative control and sample wells.
● Read results at 5 minutes. If no color is seen at 5 minutes, the sample is negative.
● Always compare results to the negative control. The kit positive is engineered to be a moderate antibody level and is used to verify proper addition of reagents and good washing technique. It should not be used to differentiate positive from negative results.
SYMBOL DESCRIPTIONS
Manufacturer
Zoetis Inc., Kalamazoo, MI 49007, USA
VLN/PCN 190/5029.00
www.zoetis.com
EC REP
SYNBIOTICS EUROPE SAS, a wholly-owned subsidiary of Zoetis, Gerland Plaza, BAT. E, 23 Rue Pierre Gilles de Gennes, 69007 Lyon, FRANCE
40016393
CPN: 1115043.2
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-05-29
Elsewhere on our site...
Wegovy
Wegovy (semaglutide) an FDA-approved weekly injection for weight loss and to reduce heart risks ...
Rybelsus
Rybelsus tablets are used to improve blood sugar control in adults with type 2 diabetes, and may ...
Ozempic
Learn about Ozempic (semaglutide) for type 2 diabetes treatment, weight management, cardiovascular ...
Zepbound
Zepbound (tirzepatide) is an FDA-approved weekly injection for weight loss and obstructive sleep ...
Trulicity
Trulicity is an injectable diabetes medicine that is used together with diet and exercise to ...
Lantus
Lantus is a long acting form of insulin used to treat type 1 or type 2 diabetes. Learn about side ...
Tresiba
Tresiba (insulin degludec) is used to treat diabetes mellitus. Includes Tresiba side effects ...
Phentermine
Phentermine is an appetite suppressant used together with diet and exercise to treat obesity. Learn ...
Semaglutide
Semaglutide is a GLP-1 agonist used for weight loss, type 2 diabetes, and reducing cardiovascular ...
