Tyzeka: Package Insert / Prescribing Info
Package insert / product label
Generic name: telbivudine
Dosage form: tablet, film coated, oral solution
Drug class: Nucleoside reverse transcriptase inhibitors (NRTIs)
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Drug Abuse and Dependence
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
- Medication Guide
Highlights of Prescribing Information
Tyzeka® (telbivudine) tablets, for oral use
Tyzeka® (telbivudine) solution, for oral use
Initial U.S. Approval: 2006
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS AND SEVERE ACUTE EXACERBATIONS OF HEPATITIS B
See full prescribing information for complete boxed warning.
-
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues (5.1).
- Severe acute exacerbations of hepatitis B have been reported in patients who discontinued anti-hepatitis B therapy, including Tyzeka. Hepatic function should be monitored closely in patients who discontinue therapy. Resumption of anti-hepatitis B therapy may be warranted (5.2).
Indications and Usage for Tyzeka
Tyzeka is an HBV nucleoside analogue reverse transcriptase inhibitor indicated for the treatment of chronic hepatitis B in adult patients with evidence of viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease (1.1).
Tyzeka Dosage and Administration
- Adults and Adolescents (16 years of age and older): 600 mg once daily, taken orally, with or without food (2.1).
- Renal Impairment: Dose adjustment required in patients with creatinine clearance less than 50 mL per min, (2.2) as follows:
| Creatinine Clearance (mL/min) | Tyzeka Dose | |
| Oral Solution
(100 mg/5 mL) | Tablet
(600 mg) |
|
| greater than or equal to 50 | 30 mL once daily | 1 tab every 24 hrs |
| 30 – 49 | 20 mL once daily | 1 tab every 48 hrs |
| less than 30 (not requiring dialysis) | 10 mL once daily | 1 tab every 72 hrs |
| End stage renal disease (ESRD) | 6 mL once daily | 1 tab every 96 hrs1 |
1When administered on hemodialysis days, administer Tyzeka after hemodialysis (2.2).
Dosage Forms and Strengths
- Tablet: 600 mg tablets.
- Oral Solution: 100 mg per 5 mL solution.
Contraindications
Combination of Tyzeka with pegylated interferon alfa-2a: Increased risk of peripheral neuropathy (4).
Warnings and Precautions
- Lactic acidosis and severe hepatomegaly with steatosis: If suspected, treatment should be suspended (5.1).
- Severe acute exacerbations of hepatitis B after discontinuation: Monitor hepatic function closely for at least several months (5.2, 6.1).
- Myopathy: Tyzeka should be interrupted if myopathy is suspected; and discontinued if confirmed. It is unknown whether risk of myopathy is increased with concomitant use of other medications associated with myopathy (5.3).
- Peripheral neuropathy: Risk increased when Tyzeka used in combination with alfa interferons, avoid concomitant use. Tyzeka should be interrupted if peripheral neuropathy is suspected; and discontinued if confirmed (4, 5.4, 7).
Adverse Reactions/Side Effects
In clinical trials, the most common adverse reactions (greater than or equal to 3%), of any severity, were: fatigue, increased creatine kinase (CK), headache, cough, diarrhea, abdominal pain, nausea, pharyngolaryngeal pain, arthralgia, pyrexia, rash, back pain, dizziness, myalgia, ALT increased, dyspepsia, insomnia, and abdominal distension (6.1). The most common adverse events resulting in Tyzeka discontinuation included increased CK, nausea, diarrhea, fatigue, myalgia, and myopathy (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2013
Full Prescribing Information
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS AND SEVERE ACUTE EXACERBATIONS OF HEPATITIS B
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination with antiretrovirals [see Warnings and Precautions (5.1)].
Severe acute exacerbations of hepatitis B have been reported in patients who have discontinued anti-hepatitis B therapy, including Tyzeka. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue anti-hepatitis B therapy. If appropriate, resumption of anti-hepatitis B therapy may be warranted [see Warnings and Precautions (5.2)].
1. Indications and Usage for Tyzeka
1.1 Chronic Hepatitis B
Tyzeka is indicated for the treatment of chronic hepatitis B in adult patients with evidence of viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease.
The following points should be considered when initiating therapy with Tyzeka:
- This indication is based on virologic, serologic, biochemical and histologic responses in nucleoside treatment naïve adult patients with HBeAg positive and HBeAg negative chronic hepatitis B with compensated liver disease [see Clinical Studies (14)].
- For HBeAg-positive patients, Tyzeka should only be initiated in patients with HBV DNA less than 9 log10 copies per mL and ALT greater than or equal to 2x Upper Limit of Normal (ULN) prior to treatment.
- For HBeAg-negative patients, Tyzeka should only be initiated in patients with HBV DNA less than 7 log10 copies per mL prior to treatment.
- On-treatment response should guide continued therapy [see Dosage and Administration (2.1) and Microbiology (12.4)].
- Tyzeka has not been evaluated in patients co-infected with HIV, HCV, or HDV.
- Tyzeka has not been evaluated in liver transplant recipients or in patients with decompensated liver disease.
- Tyzeka has not been studied in well-controlled trials for the treatment of patients with established nucleoside analog reverse transcriptase inhibitor-resistant hepatitis B virus infection, but is expected to be cross-resistant to lamivudine [see Microbiology (12.4)].
- The safety and efficacy of Tyzeka have not been evaluated in Black/African American or Hispanic patients [see Use in Specific Populations (8.9)].
2. Tyzeka Dosage and Administration
2.1 Adults and Adolescents (16 years of age and older)
Due to higher rates of resistance that may develop with longer term treatment among patients with incomplete viral suppression, treatment should only be initiated, if pre-treatment HBV DNA and ALT measurements are known, in the following patient populations:
For HBeAg-positive patients, HBV DNA should be less than 9 log10 copies per mL and ALT should be greater than or equal to 2x ULN prior to treatment with Tyzeka.
For HBeAg-negative patients, HBV DNA should be less than 7 log10 copies per mL prior to treatment with Tyzeka.
HBV DNA levels should be monitored at 24 weeks of treatment to assure complete viral suppression (HBV DNA less than 300 copies per mL). Alternate therapy should be initiated for patients who have detectable HBV DNA after 24 weeks of treatment. Optimal therapy should be guided by further resistance testing.
The recommended dose of Tyzeka for the treatment of chronic hepatitis B is 600 mg once daily, taken orally, with or without food.
Tyzeka oral solution (30 mL) may be considered for patients who have difficulty with swallowing tablets.
2.2 Renal Impairment
Tyzeka may be used for the treatment of chronic hepatitis B in patients with impaired renal function. No adjustment to the recommended dose of Tyzeka is necessary in patients whose creatinine clearance is greater than or equal to 50 mL per min. Adjustment of the total daily dose of Tyzeka oral solution or of the interval for administration of Tyzeka tablets is required in patients with creatinine clearance less than 50 mL per min including those with ESRD on hemodialysis (Table 1).
| Creatinine Clearance
(mL/min) | Tyzeka Oral Solution Dose
(5 mL = 100 mg) | Tyzeka Tablet Dose
(1 tablet = 600 mg) |
| greater than or equal to 50 | 30 mL once daily | 1 tablet every 24 hrs |
| 30-49 | 20 mL once daily | 1 tablet every 48 hrs |
| less than 30 (not requiring dialysis) | 10 mL once daily | 1 tablet every 72 hrs |
| ESRD | 6 mL once daily | 1 tablet every 96 hrs1 |
| 1When administered on hemodialysis days, Tyzeka should be administered after hemodialysis. | ||
2.3 Hepatic Impairment
No adjustment to the recommended dose of Tyzeka is necessary in patients with hepatic impairment.
2.4 Duration of Therapy
For patients with incomplete viral suppression (HBV DNA greater than or equal to 300 copies per mL) after 24 weeks of treatment, alternate therapy should be instituted. HBV DNA should be monitored every 6 months to assure continued response. If patients test positive for HBV DNA at any time after their initial response, alternate treatment should be instituted. Optimal therapy should be guided by resistance testing.
The optimal duration of therapy with Tyzeka for patients with chronic hepatitis B is unknown.
3. Dosage Forms and Strengths
4. Contraindications
Combination of Tyzeka with pegylated interferon alfa-2a is contraindicated because of increased risk of peripheral neuropathy [see Warnings and Precautions (5.4) and Drug Interactions (7)].
5. Warnings and Precautions
5.1 Lactic Acidosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination with antiretrovirals. Female gender, obesity, and prolonged nucleoside exposure may be risk factors. Particular caution should be exercised when administering HBV nucleoside analogue reverse transcriptase inhibitors to patients with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with Tyzeka should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
5.2 Exacerbations of Hepatitis B after Discontinuation of Treatment
Severe acute exacerbations of hepatitis B have been reported in patients who have discontinued anti-hepatitis B therapy, including Tyzeka. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue anti-hepatitis B therapy. If appropriate, resumption of anti-hepatitis B therapy may be warranted [see Adverse Reactions (6.1)].
5.3 Myopathy
Cases of myopathy/myositis have been reported with Tyzeka use several weeks to months after starting therapy. Myopathy has also been reported with some other drugs in this class. Rhabdomyolysis has been reported during postmarketing use of Tyzeka [see Adverse Reactions (6.2)].
Uncomplicated myalgia has been reported in Tyzeka-treated patients [see Adverse Reactions (6.1)]. Myopathy, defined as persistent unexplained muscle aches and/or muscle weakness in conjunction with increases in creatine kinase (CK) values, should be considered in any patient with diffuse myalgias, muscle tenderness, or muscle weakness. Among patients with Tyzeka-associated myopathy, no pattern with regard to the degree or timing of CK elevations has been observed. In addition, the predisposing factors for the development of myopathy among Tyzeka recipients are unknown. Patients should be advised to report promptly unexplained muscle aches, pain, tenderness, or weakness. Tyzeka therapy should be interrupted if myopathy is suspected, and discontinued if myopathy is confirmed. It is unknown whether the risk of myopathy during treatment with drugs in this class is increased with concurrent administration of other drugs associated with myopathy, including but not limited to: corticosteroids, chloroquine, hydroxychloroquine, certain HMGCoA reductase inhibitors, fibric acid derivatives, penicillamine, zidovudine, cyclosporine, erythromycin, niacin, and certain azole antifungals. Physicians initiating concomitant treatment with any drug associated with myopathy should monitor patients closely for any signs or symptoms of unexplained muscle pain, tenderness, or weakness.
5.4 Peripheral Neuropathy
Peripheral neuropathy has been reported with Tyzeka alone or in combination with pegylated interferon alfa-2a and other interferons. In one clinical trial, an increased risk and severity of peripheral neuropathy was observed with the combination use of Tyzeka 600mg daily and pegylated interferon alfa-2a 180 micrograms once weekly compared to Tyzeka or pegylated interferon alfa-2a alone [see Contraindications (4) and Drug Interactions (7)]. Such risk cannot be excluded for other dose regimens of pegylated interferon alfa-2a, or other alfa interferons (pegylated or standard). The safety and efficacy of Tyzeka in combination with pegylated interferons or other interferons for the treatment of chronic hepatitis B have not been demonstrated. Patients should be advised to report any numbness, tingling, and/or burning sensations in the arms and/or legs, with or without gait disturbance. Tyzeka therapy should be interrupted if peripheral neuropathy is suspected, and discontinued if peripheral neuropathy is confirmed [see Adverse Reactions (6.1)].
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in other sections of the labeling:
- Lactic acidosis and severe hepatomegaly with steatosis [see Boxed Warning, Warnings and Precautions (5.1)]
- Severe acute exacerbations of hepatitis after discontinuation of treatment [see Boxed Warning, Warnings and Precautions (5.2)]
- Myopathy [see Warnings and Precautions (5.3)]
- Peripheral Neuropathy [see Warnings and Precautions (5.4)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Assessment of adverse reactions is primarily based on two trials (007 GLOBE and NV-02B-015) in which 1,699 subjects with chronic hepatitis B received double-blind treatment with Tyzeka 600 mg per day (n=847 subjects) or lamivudine (n=852 subjects) for 104 weeks. The median duration of therapy was 104 weeks for both treatment groups.
In the 104 week clinical trials, most adverse experiences reported with Tyzeka were classified as mild or moderate in severity and were not attributed to Tyzeka. Selected adverse events of any severity which were reported in greater than or equal to 3% of Tyzeka and lamivudine recipients are shown in Table 2. With the exception of increased creatine kinase (CK), which was reported more frequently among Tyzeka recipients, the adverse event profile was similar for the two drugs.
| Adverse Event (Preferred Term) | Tyzeka
N=847 n (%)b | Lamivudine
N=852 n (%)b |
| Fatigue | 106 (13) | 95 (11) |
| CK increased | 90 (11) | 52 (6) |
| Headache | 83 (10) | 95 (11) |
| Cough | 52 (6) | 45 (5) |
| Diarrhea | 50 (6) | 46 (5) |
| Abdominal pain, upper | 49 (6) | 52 (6) |
| Nausea | 45 (5) | 40 (5) |
| Pharyngolaryngeal pain | 38 (5) | 31 (4) |
| Arthralgia | 37 (4) | 38 (5) |
| Pyrexia | 34 (4) | 27 (3) |
| Rash | 33 (4) | 21 (3) |
| Back pain | 33 (4) | 32 (4) |
| Dizziness | 32 (4) | 43 (5) |
| Abdominal pain | 29 (3) | 31 (4) |
| Myalgia | 27 (3) | 17 (2) |
| ALT increased | 27 (3) | 31 (4) |
| Dyspepsia | 24 (3) | 39 (5) |
| Insomnia | 24 (3) | 22 (3) |
| Abdominal distension | 22 (3) | 19 (2) |
| Pruritus | 18 (2) | 23 (3) |
| Hepatitis B exacerbation | 17 (2) | 36 (4) |
| a Adverse events reported in greater than or equal to 3% subjects in either treatment group b n (%) = the number and proportion of subjects in whom adverse event was reported |
||
Moderate to severe (Grade 2-4) adverse events were reported in 239/847 (28%) of Tyzeka recipients and 229/852 (27%) of lamivudine recipients. The profile of adverse events of moderate to severe intensity was similar in both treatment groups and no individual adverse event was reported in greater than 2% of subjects in either treatment group.
Discontinuations due to adverse events were reported in 4% of Tyzeka recipients and 4% of lamivudine recipients. The most common adverse events resulting in Tyzeka discontinuation included increased CK, nausea, diarrhea, fatigue, myalgia, and myopathy.
Peripheral neuropathy was reported as an adverse event in less than 1% (2/847) of subjects receiving Tyzeka monotherapy [see Warnings and Precautions (5.4)]. Of Tyzeka-treated subjects less than 1% (5/847) were diagnosed with myopathy/myositis (presenting with muscular weakness) [see Warnings and Precautions (5.3)].
Laboratory Abnormalities
Frequencies of selected treatment-emergent laboratory abnormalities in the 007 GLOBE and NV-02B-015 trials are listed in Table 3.
| Test | Tyzeka
600 mg (n=847) | Lamivudine
100 mg (n=852) |
| Creatine Kinase (CK) greater than 7.0 x ULN | 13% | 4% |
| ALT greater than 10.0 x ULN and 2.0 x baselineb | 5% | 8% |
| ALT greater than 3 x baseline | 7% | 13% |
| AST (SGOT) greater than 3.0 x baseline | 6% | 10% |
| Lipase greater than 2.5 x ULN | 2% | 4% |
| Amylase greater than 3.0 x ULN | less than 1% | less than 1% |
| Total Bilirubin greater than 5.0 x ULN | less than 1% | less than 1% |
| Neutropenia (ANC less than or equal to 749/mm3) | 2% | 2% |
| Thrombocytopenia (Platelets less than or equal to 49,999/mm3) | less than 1% | less than 1% |
| a On-treatment value worsened from baseline to Grade 3 or Grade 4 during therapy | ||
| b American Association for the Study of Liver Diseases (AASLD) definition of acute hepatitis flare | ||
Creatine Kinase (CK) Elevations
Creatine kinase (CK) elevations were more frequent among subjects on Tyzeka treatment. By 104 weeks of treatment, Grade 1-4 CK elevations occurred in 79% of Tyzeka-treated subjects and 47% of lamivudine-treated subjects. Grade 3 or 4 CK elevations occurred in 13% of Tyzeka-treated subjects and 4% of lamivudine-treated subjects. Most CK elevations were asymptomatic, but the mean recovery time was longer for subjects on Tyzeka than subjects on lamivudine.
Among Tyzeka-treated subjects with Grade 1-4 CK elevations, 10% developed a musculoskeletal adverse event compared to 5% of lamivudine-treated subjects. A total of 2% (13/847) Tyzeka-treated subjects interrupted or discontinued trial drug due to CK elevation or musculoskeletal adverse events1.
______________________
1 Includes the Preferred Terms: back pain, chest wall pain, non-cardiac chest pain, chest discomfort, flank pain, muscle cramp, muscular weakness, musculoskeletal pain, musculoskeletal chest pain, musculoskeletal discomfort, musculoskeletal stiffness, myalgia, myofascial pain syndrome, myopathy, myositis, neck pain, and pain in extremity.
______________________
ALT Flares During Treatment
The incidence of ALT flares, defined as ALT greater than 10 x ULN and greater than 2 x baseline, was similar in the two treatment arms (3%) in the first six months. After week 24, ALT flares were reported less frequently in the Tyzeka arm (2%) compared to the lamivudine arm (5%). Periodic monitoring of hepatic function is recommended during chronic hepatitis B treatment.
Exacerbations of Hepatitis after Discontinuation of Treatment
In the subset of subjects who discontinued treatment prematurely for reasons other than efficacy, or who elected not to continue Tyzeka in another clinical trial, 9/154 (6%) Tyzeka-treated and 10/180 (6%) lamivudine-treated subjects experienced an exacerbation of hepatitis (ALT elevation greater than 2 x baseline and greater than 10 x ULN) in the 4-month post-treatment period.
Results at 208 Weeks
After 104 weeks of blinded therapy in trials 007 GLOBE and NV-02B-015, 667 subjects received Tyzeka in an open-label extension trial, CLDT600A2303. Of those initially randomized to Tyzeka therapy, 78% of subjects (530/680) from trial 007 GLOBE and 82% (137/167) of subjects from trial NV-02B-015 enrolled into the extension trial and continued Tyzeka treatment for up to 208 weeks. The long-term Tyzeka safety population in trial CLDT600A2303 consisted of 655 subjects, including 518 subjects from trial 007 GLOBE and 137 subjects from trial NV-02B-015.
The overall safety profile from the pooled analysis up to 104 and 208 weeks was similar. Grade 3/4 CK elevations occurred in 16% of subjects (104/655) treated with Tyzeka in trial CLDT600A2303. Most grade 3/4 CK elevations were asymptomatic (74% of subjects without any muscle related adverse reaction) and transient (98% of episodes lasted one or two visits (visit interval 2 - 12 weeks) and 87% of subjects had one or two episodes). Most grade 3/4 CK elevations (93%) resolved spontaneously or returned to baseline levels. Two cases of myopathy and two cases of myositis were reported in the 655 Tyzeka-treated subjects.
Among the cohort of 655 subjects continuing Tyzeka for up to 208 weeks in trial CLDT600A2303, including the subgroup of patients (n=223) with mild renal impairment (eGFR 60-90 mL per min) at baseline, mean estimated GFR assessed by MDRD did not decline.
6.2 Postmarketing Experience
The following adverse reactions have been reported during post approval use of Tyzeka. Because these reactions were reported voluntarily from a population of unknown size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Musculoskeletal and Connective Tissue Disorders
Rhabdomyolysis
Nervous System Disorders
Paraesthesia, hypoaesthesia
Metabolism and Nutrition Disorders
Lactic acidosis
Related/similar drugs
7. Drug Interactions
Tyzeka is excreted mainly by passive diffusion so the potential for interactions between Tyzeka and other drugs eliminated by renal excretion is low. However, because Tyzeka is eliminated primarily by renal excretion, coadministration of Tyzeka with drugs that alter renal function may alter plasma concentrations of Tyzeka [see Clinical Pharmacology (12.3)].
A clinical trial investigating the combination of Tyzeka, 600 mg daily, with pegylated interferon alfa-2a, 180 micrograms once weekly by subcutaneous administration, indicates that this combination is associated with an increased risk of peripheral neuropathy occurrence and severity, in comparison to Tyzeka or pegylated interferon alfa-2a alone [see Contraindications (4) and Warnings and Precautions (5.4)].
8. Use In Specific Populations
8.1 Pregnancy
Category B: Telbivudine is not teratogenic and has shown no adverse effects in developing embryos and fetuses in preclinical studies. Studies in pregnant rats and rabbits showed that telbivudine crosses the placenta. Developmental toxicity studies revealed no evidence of harm to the fetus in rats and rabbits at doses up to 1000 mg per kg per day, providing exposure levels 6- and 37-times higher, respectively, than those observed with the 600 mg per day dose in humans.
There are no adequate and well-controlled trials of Tyzeka in pregnant women. Because animal reproductive toxicity studies are not always predictive of human response, Tyzeka should be used during pregnancy only if potential benefits outweigh the risks.
Pregnancy Registry: To monitor fetal outcomes of pregnant women exposed to Tyzeka, healthcare providers are encouraged to register such patients in the Antiretroviral Pregnancy Registry by calling 1-800-258-4263.
8.2 Labor and Delivery
There are no trials in pregnant women and no data on the effect of Tyzeka on transmission of HBV from mother to infant. Therefore, appropriate interventions should be used to prevent neonatal acquisition of HBV infection.
8.3 Nursing Mothers
Telbivudine is excreted in the milk of rats. It is not known whether Tyzeka is excreted in human milk. Mothers should be instructed not to breast-feed if they are receiving Tyzeka.
8.4 Pediatric Use
Safety and effectiveness of Tyzeka in pediatric patients have not been established.
8.5 Geriatric Use
Clinical trials of Tyzeka did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently from younger subjects. In general, caution should be exercised when prescribing Tyzeka to elderly patients, considering the greater frequency of decreased renal function due to concomitant disease or other drug therapy. Renal function should be monitored in elderly patients, and dosage adjustments should be made accordingly [see Clinical Pharmacology (12.3) and Dosage and Administration (2.2)].
8.6 Renal Impairment
Tyzeka is eliminated primarily by renal excretion, therefore dose regimen adjustment is recommended in patients with creatinine clearance less than 50 mL per min, including patients with ESRD requiring hemodialysis [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
8.7 Liver Transplant Recipients
The safety and efficacy of Tyzeka in liver transplant recipients have not been evaluated. The steady-state pharmacokinetics of Tyzeka was not altered following multiple dose administration in combination with cyclosporine. If Tyzeka treatment is determined to be necessary for a liver transplant recipient who has received or is receiving an immunosuppressant that may affect renal function, such as cyclosporine or tacrolimus, renal function should be monitored both before and during treatment with Tyzeka [see Clinical Pharmacology (12.3) and Dosage and Administration (2.2)].
9. Drug Abuse and Dependence
Tyzeka is not a controlled substance and no potential for dependence has been observed.
10. Overdosage
There is no information on intentional overdose of Tyzeka, but one subject experienced an unintentional and asymptomatic overdose. Healthy subjects who received Tyzeka doses up to 1800 mg per day for 4 days had no increase in or unexpected adverse events. A maximum tolerated dose for Tyzeka has not been determined. In the event of an overdose, Tyzeka should be discontinued, the patient must be monitored for evidence of toxicity, and appropriate general supportive treatment applied as necessary.
In case of overdosage, hemodialysis may be considered. Within 2 hours, following a single 200 mg dose of telbivudine, a 4-hour hemodialysis session removed approximately 23% of the telbivudine dose.
11. Tyzeka Description
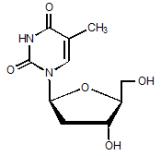
Tyzeka is the trade name for telbivudine, a synthetic thymidine nucleoside analogue with activity against hepatitis B virus (HBV). The chemical name for telbivudine is 1-((2S,4R,5S)-4-hydroxy-5-hydroxymethyltetrahydrofuran-2-y1)-5-methyl-1H-pyrimidine-2,4-dione, or 1-(2-deoxy-β-L-ribofuranosyl)-5-methyluracil. Telbivudine is the unmodified β-L enantiomer of the naturally occurring nucleoside, thymidine. Its molecular formula is C10H14N2O5, which corresponds to a molecular weight of 242.23. Telbivudine has the following structural formula:
Telbivudine is a white to slightly yellowish powder. Telbivudine is sparingly soluble in water (greater than 20 mg per mL), and very slightly soluble in absolute ethanol (0.7 mg per mL) and n-octanol (0.1 mg per mL).
Tyzeka film-coated tablets are available for oral administration in 600 mg strength. Tyzeka 600 mg film-coated tablets contain the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, povidone, and sodium starch glycolate. The tablet coating contains titanium dioxide, polyethylene glycol, talc, and hypromellose.
Tyzeka oral solution is available for oral administration in 100 mg per 5 mL strength. Tyzeka oral solution contains the following inactive ingredients: citric acid anhydrous, benzoic acid, passion fruit flavor, sodium saccharin, sodium hydroxide, and purified water. A 600 mg dose (30 mL) of Tyzeka oral solution contains approximately 47 mg of sodium.
12. Tyzeka - Clinical Pharmacology
12.2 Pharmacodynamics
In a randomized, partially single-blinded, placebo and active-controlled, four-period crossover trial, 53 healthy subjects were administered Tyzeka 600 mg, a supratherapeutic Tyzeka 1800 mg dose, placebo, and moxifloxacin 400 mg. After 7 days of dosing, Tyzeka did not prolong the QT interval. The maximum placebo-adjusted mean (upper 1-side 95% CI) change from baseline in QTcF on day 7 were 3.4 msec (5.9 msec) for the 600 mg and 4.4 msec (6.9 msec) for the 1800 mg dosing regimens.
12.3 Pharmacokinetics in Adults
The single- and multiple-dose pharmacokinetics of Tyzeka were evaluated in healthy subjects and in patients with chronic hepatitis B. Tyzeka pharmacokinetics are similar between both populations.
Absorption and Bioavailability
Following oral administration of Tyzeka 600 mg once-daily in healthy subjects (n=12), steady state peak plasma concentration (Cmax) was 3.69 ± 1.25 microgram per mL (mean ± SD) which occurred between 1 and 4 hours (median 2 hours), AUC was 26.1 ± 7.2 microgram * hour per mL (mean ± SD), and trough plasma concentrations (Ctrough) were approximately 0.2-0.3 microgram per mL. Steady state was achieved after approximately 5 to 7 days of once-daily administration with ~1.5-fold accumulation, suggesting an effective half-life of ~15 hours.
Effects of Food on Oral Absorption
Tyzeka absorption and exposure were unaffected when a single 600 mg dose was administered with a high-fat (~55 g), high-calorie (~950 kcal) meal. Tyzeka may be taken with or without food.
Distribution
In vitro binding of telbivudine to human plasma proteins is low (3.3%). After oral dosing, the estimated apparent volume of distribution is in excess of total body water, suggesting that telbivudine is widely distributed into tissues. Telbivudine was equally partitioned between plasma and blood cells.
Metabolism and Elimination
No metabolites of telbivudine were detected following administration of [14C]-telbivudine in humans. Telbivudine is not a substrate, or inhibitor of the cytochrome P450 (CYP450) enzyme system.
After reaching the peak concentration, plasma concentrations of Tyzeka declined in a biexponential manner with a terminal elimination half-life (T1/2) of 40-49 hours. Tyzeka is eliminated primarily by urinary excretion of unchanged drug. The renal clearance of Tyzeka approaches normal glomerular filtration rate suggesting that passive diffusion is the main mechanism of excretion. Approximately 42% of the dose is recovered in the urine over 7 days following a single 600 mg oral dose of Tyzeka. Because renal excretion is the predominant route of elimination, patients with moderate to severe renal dysfunction and those undergoing hemodialysis require a dose regimen adjustment [see Dosage and Administration (2.2)].
Special Populations
Gender: There are no significant gender-related differences in Tyzeka pharmacokinetics.
Race: There are no significant race-related differences in Tyzeka pharmacokinetics.
Pediatrics and Geriatrics: Pharmacokinetic studies have not been conducted in children or elderly subjects.
Renal Impairment: Single-dose pharmacokinetics of Tyzeka have been evaluated in subjects (without chronic hepatitis B) with various degrees of renal impairment (as assessed by creatinine clearance). Based on the results shown in Table 4, adjustment of the dose regimen for Tyzeka is recommended in patients with creatinine clearance of less than 50 mL per min [see Dosage and Administration (2.2)].
| Renal Function (Creatinine Clearance in mL/min) | |||||
| Normal
(greater than 80) (n=8) 600 mg | Mild (50-80)
(n=8) 600 mg | Moderate (30-49)
(n=8) 400 mg | Severe (less than 30)
(n=6) 200 mg | ESRD/Hemodialysis
(n=6) 200 mg |
|
| Cmax (μg/mL) | 3.4±0.9 | 3.2±0.9 | 2.8±1.3 | 1.6±0.8 | 2.1±0.9 |
| AUC0-INF (μg•hr/mL) | 28.5±9.6 | 32.5±10.1 | 36.0±13.2 | 32.5±13.2 | 67.4±36.9 |
| CLRENAL (L/hr) | 7.6±2.9 | 5.0±1.2 | 2.6±1.2 | 0.7±0.4 | |
Renally Impaired Subjects on Hemodialysis: Hemodialysis (up to 4 hours) reduces systemic Tyzeka exposure by approximately 23%. Following dose regimen adjustment for creatinine clearance [see Dosage and Administration (2.2)], no additional dose modification is necessary during routine hemodialysis. When administered on hemodialysis days, Tyzeka should be administered after hemodialysis.
Hepatic Impairment: The pharmacokinetics of Tyzeka following a single 600 mg dose have been studied in subjects (without chronic hepatitis B) with various degrees of hepatic impairment. There were no changes in Tyzeka pharmacokinetics in hepatically impaired subjects compared to unimpaired subjects. Results of these studies indicate that no dosage adjustment is necessary for patients with hepatic impairment.
Drug Interactions
Drug-drug interaction studies show that lamivudine, adefovir dipivoxil, cyclosporine, pegylated interferon alfa-2a, and tenofovir disoproxil fumarate do not alter Tyzeka pharmacokinetics. In addition, Tyzeka does not alter the pharmacokinetics of lamivudine, adefovir dipivoxil, cyclosporine, or tenofovir disoproxil fumarate. No definitive conclusion could be drawn regarding the effects of Tyzeka on the pharmacokinetics of pegylated interferon alfa-2a due to the high inter-individual variability of pegylated interferon alfa-2a concentrations. At concentrations up to 12 times that in humans, telbivudine did not inhibit in vitro metabolism mediated by any of the following human hepatic microsomal cytochrome P450 (CYP) isoenzymes known to be involved in human medicinal product metabolism: 1A2, 2C9, 2C19, 2D26, 2E1, and 3A4. Based on the above results and the known elimination pathway of telbivudine, the potential for CYP450-mediated interactions involving telbivudine with other medicinal products is low.
12.4 Microbiology
Mechanism of Action
Telbivudine is a synthetic thymidine nucleoside analogue with activity against HBV DNA polymerase. It is phosphorylated by cellular kinases to the active triphosphate form, which has an intracellular half-life of 14 hours. Telbivudine 5'-triphosphate inhibits HBV DNA polymerase (reverse transcriptase) by competing with the natural substrate, thymidine 5'-triphosphate. Incorporation of telbivudine 5'-triphosphate into viral DNA causes DNA chain termination. Telbivudine is an inhibitor of both HBV first strand (EC50 value = 1.3 ± 1.6 micromolar) and second strand synthesis (EC50 value = 0.2 ± 0.2 micromolar). Telbivudine 5'-triphosphate at concentrations up to 100 micromolar did not inhibit human cellular DNA polymerases α, β, or γ. No appreciable mitochondrial toxicity was observed in HepG2 cells treated with telbivudine at concentrations up to 10 micromolar.
Antiviral Activity
The antiviral activity of telbivudine was assessed in the HBV-expressing human hepatoma cell line 2.2.15, as well as in primary duck hepatocytes infected with duck hepatitis B virus. The concentration of telbivudine that effectively inhibited 50% of viral DNA synthesis (EC50) in both systems was approximately 0.2 micromolar. The anti-HBV activity of telbivudine was additive with adefovir in cell culture, and was not antagonized by the HIV NRTIs didanosine and stavudine. Telbivudine was not antagonistic to the anti-HIV activity of abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, or zidovudine. Transient reductions in HIV-1 RNA have been seen in some patients after administration of telbivudine in the absence of antiretroviral therapy. The clinical significance of these reductions has not been determined.
Resistance
Trial NV-02B-007 (007 GLOBE): In an as-treated analysis of the Phase III global registration trial, 59% (251/429) of treatment-naïve HBeAg-positive and 89% (202/227) of treatment-naïve HBeAg-negative subjects receiving Tyzeka 600 mg once daily achieved undetectable serum HBV DNA levels (less than 300 copies per mL) by Week 52. Of those who continued treatment beyond Week 52, 58% (243/418) and 85% (190/224) of HBeAg-positive and HBeAg-negative Tyzeka recipients, respectively, had undetectable HBV DNA at Week 104 (or at the end of dosing in treatment Year 2).
Genotypic analysis of paired baseline and treatment failure isolates from 181 evaluable subjects with amplifiable HBV DNA and greater than or equal to 16 weeks of Tyzeka treatment showed that the rtM204I/V substitution was associated with virologic failure (HBV DNA greater than or equal to 1,000 copies per mL) and virologic rebound (HBV DNA greater than or equal to 1 log10 increase above nadir). The rtM204I/V substitution was detectable in isolates from 78% (142/181) of evaluable subjects, and was frequently found with substitutions rtL80I/V and rtL180M. The rtM204I/V substitution was found infrequently with rtV27A, rtL82M, rtV173L, rtT184I/S, rtA200V, rtL229F/V/W, and rtR289K substitutions. The HBV of 16 subjects developed rtA181S/T amino acid substitutions while receiving Tyzeka. Eight of these 16 subjects had outgrowth of HBV expressing an rtM204I/V substitution without the rtA181 substitution and 1 subject’s HBV had both the rtM204I and rtA181T substitutions.
Trial CLDT600A2303: After 2 years of Tyzeka monotherapy in the 007 GLOBE trial, 77% (505/656) of subjects entered the open-label CLDT600A2303 extension trial to continue Tyzeka for up to 2 additional years, including 349 subjects who had undetectable levels of HBV DNA and 156 subjects who were viremic at entry. The rtM204I/V substitution was detectable in the virus from 83% (39/47) of the subjects losing viral suppression and having evaluable genotypic data. Of evaluable viremic subjects entering the extension, 25/33 (76%) developed rtM204I/V substitutions. Overall, 64 subjects developed genotypic resistance to Tyzeka with evidence of emerging rtM204I/V substitutions during the 2 years of Tyzeka treatment in this extension trial.
Subjects with higher baseline viral load had higher rates of genotypic resistance to Tyzeka, while subjects who achieved HBV DNA levels less than 300 copies per mL at Week 24 had lower rates of genotypic resistance to Tyzeka. The cumulative frequency of genotypic resistance (emergence of the rtM204I/V substitution) to Tyzeka in nucleos(t)ide treatment-naïve subjects was 7% and 22% at Weeks 52 and 104 of the controlled 007 GLOBE trial, and 30% and 35% at Weeks 156 and 208 of the open-label extension trial (CLDT600A2303), respectively (Table 5).
One-hundred-sixty-seven subjects (25% of those in the 007 GLOBE trial) were treated with Tyzeka according to current dosing recommendations [see Indications and Usage (1.1)]. Eighty-four percent (140/167) of these subjects qualified at 24 weeks for continued Tyzeka treatment (HBV DNA less than 300 copies per mL). Retrospective calculation of the cumulative rate of genotypic resistance to Tyzeka for this subgroup of subjects was 0%, 3%, 12%, and 16% at Weeks 52, 104, 156, and 208, respectively (Table 5).
| Trial | Cumulative genotypic resistance rate1 | ||
| Overall study population | Subjects treated with Tyzeka according to current dosing recommendations2 | ||
| NV-02B-007 (007 GLOBE trial) | Week 52 | 7% | 0% |
| Week 104 | 22% | 3% | |
| CLDT600A2303 (104-week extension trial) | Week 156 | 30% | 12% |
| Week 208 | 35% | 16% | |
| 1The cumulative rates of genotypic resistance to Tyzeka were calculated using the formula previously described by Pawlotsky et al. (2008). | |||
| 2Tyzeka dosing recommendations are provided in this Package Insert [see Indications and Usage (1.1) and Dosage and Administration (2.1)]. | |||
Cross-Resistance
Cross-resistance has been observed among HBV nucleos(t)ide analogues. In cell-based assays, lamivudine-resistant HBV strains expressing either the rtM204I substitution or the rtL180M/rtM204V double substitution had greater than or equal to 1,000-fold reduced susceptibility to telbivudine. Telbivudine retained wild-type phenotypic activity (1.2-fold reduction) against HBV expressing rtM204V alone. Most subjects (92%, 155/169) whose virus developed lamivudine resistance-associated substitutions (rtM204I/V) during 2 years of lamivudine treatment in the 007 GLOBE trial remained viremic (HBV DNA greater than 300 copies per mL) after up to 2 years of Tyzeka monotherapy in the CLDT600A2303 extension trial, including 91% (50/55) of the subjects with the rtM204V substitution.
HBV encoding the adefovir resistance-associated substitution rtA181V showed 3- to 5-fold reduced susceptibility to telbivudine in cell culture. The rtA181S and rtA181T substitutions conferred 2.7- and 3.5-fold reductions in susceptibility to telbivudine, respectively. The rtA181T substitution is associated with decreased clinical response in subjects with HBV treated with adefovir and entecavir. HBV encoding the adefovir resistance-associated substitution rtN236T remained susceptible to telbivudine.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Telbivudine has shown no carcinogenic potential. Long term oral carcinogenicity studies with telbivudine were negative in mice and rats at exposures up to 14 times those observed in humans at the therapeutic dose of 600 mg per day.
There was no evidence of genotoxicity based on in vitro or in vivo tests. Telbivudine was not mutagenic in the Ames bacterial reverse mutation assay using S. typhimurium and E. coli strains with or without metabolic activation. Telbivudine was not clastogenic in mammalian-cell gene mutation assays, including human lymphocyte cultures and an assay with Chinese hamster ovary cells with or without metabolic activation. Furthermore, telbivudine showed no effect in an in vivo micronucleus study in mice.
Effects on fertility were studied in rats administered telbivudine as juveniles or adults. Juvenile rats were treated with telbivudine at doses of 0, 250, 1000, and 2000 mg per kg per day from post natal days 14 to 70. These rats were mated following a 5 week drug-free recovery period. Up to 50% reduction of fertility was associated with doses 1000 mg per kg per day and higher, which was equivalent to a systemic exposure approximately 7.5 times that achieved in humans at the therapeutic dose. The no observed adverse effect level (NOAEL) for effects on fertility or mating parameters was 250 mg per kg per day, which was equivalent to systemic exposure levels 2.5 to 2.8 times that achieved in humans at the therapeutic dose. In contrast, such reduction of fertility was absent in adult rats treated with telbivudine at doses up to 2000 mg per kg per day, equivalent to a systemic exposure approximately 14 times that achieved in humans at the therapeutic dose.
14. Clinical Studies
14.1 Clinical Experience in Nucleoside-Naïve Adults
The safety and efficacy of long-term (104-week) Tyzeka treatment were evaluated in one active-controlled, clinical trial (NV-02B-007 GLOBE Trial) that included 1,367 subjects with chronic hepatitis B and a smaller supportive trial (NV-02B-015) that included 332 subjects. Subjects were 16 years of age or older, with chronic hepatitis B, evidence of HBV infection with viral replication (HBsAg-positive, HBeAg-positive or HBeAg-negative, HBV DNA detectable by a PCR assay), and elevated ALT levels greater than or equal to 1.3 x ULN, no evidence of hepatic decompensation, and chronic inflammation on liver biopsy compatible with chronic viral hepatitis.
NV-02B-007 GLOBE Trial
The Week 52 and Week 104 results of the 007 GLOBE trial are summarized below.
The 007 GLOBE trial was a Phase III, randomized, double-blind, multinational trial of Tyzeka 600 mg once daily compared to lamivudine 100 mg once daily for a treatment period of 104 weeks in 1,367 (n= 680 Tyzeka; n=687 lamivudine) nucleoside-naïve chronic hepatitis B HBeAg-positive and HBeAg-negative subjects. The primary data analysis was conducted after all subjects had reached Week 52.
HBeAg-positive Subjects: (n= 458 Tyzeka; n= 463 lamivudine) The mean age of subjects was 32 years, 74% were male, 82% were Asian, 12% were Caucasian, and 6% had previously received alfa-interferon therapy. At baseline, subjects had a mean Knodell Necroinflammatory Score greater than or equal to 7; mean serum HBV DNA as measured by Roche COBAS Amplicor® PCR assay was 9.52 log10 copies per mL; and mean serum ALT was 153 IU per L. Pre- and post-liver biopsy samples were adequate for 86% of subjects.
HBeAg-negative Subjects: (n=222 Tyzeka; n= 224 lamivudine)The mean age of subjects was 43 years, 77% were male, 65% were Asian, 23% were Caucasian, and 11% had previously received alfa-interferon therapy. At baseline, subjects had a mean Knodell Necroinflammatory Score greater than or equal to 7; mean serum HBV DNA as measured by Roche COBAS Amplicor® PCR assay was 7.54 log10 copies per mL; and mean serum ALT was 140 IU per L. Pre- and post-liver biopsy samples were adequate for 92% of subjects.
Clinical Results
Clinical and virologic efficacy endpoints were evaluated separately in the HBeAg-positive and HBeAg-negative subject populations.
The primary endpoint of Therapeutic Response at Week 52 was a composite endpoint requiring suppression of HBV DNA to less than 5 log10 copies per mL in conjunction with either loss of serum HBeAg or ALT normalization. Key secondary endpoints included histologic response, ALT normalization, and measures of virologic response.
At Week 52, in HBeAg-positive subjects, 75% of Tyzeka subjects and 67% of lamivudine subjects had a Therapeutic Response; in HBeAg-negative subjects, 75% of Tyzeka subjects and 77% of lamivudine subjects had a Therapeutic Response.
Analysis of the histological response at Week 52 is shown in Table 6.
| HBeAg-positive (n=797) | HBeAg-negative (n=417) | |||
| Tyzeka
600 mg (n=399)1 | Lamivudine
100 mg (n=398)1 | Tyzeka
600 mg (n=205)1 | Lamivudine
100 mg (n=212)1 |
|
| Histologic Response2 | ||||
| Improvement | 69% | 60% | 69% | 68% |
| No Improvement | 19% | 26% | 23% | 25% |
| Missing Week 52 Biopsy | 12% | 15% | 8% | 7% |
| Ishak Fibrosis Score3 | ||||
| Improvement | 41% | 46% | 48% | 44% |
| No Change | 39% | 32% | 34% | 43% |
| Worsening | 9% | 7% | 10% | 5% |
| Missing Week 52 Biopsy | 12% | 15% | 8% | 7% |
| 1 Subjects with greater than or equal to one dose of trial drug with evaluable baseline liver biopsies and baseline Knodell Necroinflammatory Score greater than or equal to 2 | ||||
| 2 Histologic Response defined as greater than or equal to 2 point decrease in Knodell Necroinflammatory Score from baseline with no worsening of the Knodell Fibrosis Score | ||||
| 3 For Ishak Fibrosis Score, improvement defined as greater than or equal to a 1-point reduction in Ishak Fibrosis Score from baseline to Week 52 | ||||
Subjects were eligible to continue blinded treatment to Week 104. In the ITT population, 624/680 (92%) Tyzeka recipients and 599/687 (87%) lamivudine recipients completed trial treatment to Week 104. At Week 104, in HBeAg-positive subjects, 63% of Tyzeka subjects and 48% of lamivudine subjects had a Therapeutic Response, while in HBeAg-negative subjects 78% of Tyzeka subjects and 66% of lamivudine subjects had a Therapeutic Response.
Selected virologic, biochemical, and serologic outcome measures at Weeks 52 and 104 are shown in Table 7.
|
Response Parameter | HBeAg-positive (n=921) | HBeAg-negative (n=446) | ||||||
| Tyzeka
600 mg (n=458) | Lamivudine
100 mg (n=463) | Tyzeka
600 mg (n=222) | Lamivudine
100 mg (n=224) |
|||||
| Week 52 | Week 104 | Week 52 | Week 104 | Week 52 | Week 104 | Week 52 | Week 104 | |
| Mean HBV DNA Reduction from Baseline (log10 copies/mL) ± SEM1 |
-6.45 (0.11) | -5.74 (0.15) |
-5.54 (0.11) | -4.42 (0.15) |
-5.23 (0.13) | -5.00 (0.15) |
-4.40 (0.13) | -4.17 (0.16) |
| % Subjects HBV DNA undetectable by PCR | 60% | 56% | 40% | 39% | 88% | 82% | 71% | 57% |
| ALT Normalization2 | 77% | 70% | 75% | 62% | 74% | 78% | 79% | 70% |
| HBeAg Seroconversion3 | 23% | 30% | 22% | 25% | NA | NA | NA | NA |
| HBeAg Loss3 | 26% | 35% | 23% | 29% | NA | NA | NA | NA |
| 1 Roche COBAS Amplicor® Assay (LLOQ less than or equal to 300 copies/mL). 2ALT normalization assessed only in subjects with ALT greater than ULN at baseline. |
||||||||
| 3 HBeAg seroconversion and loss assessed only in subjects with detectable HBeAg at baseline. | ||||||||
Subjects who achieved non-detectable HBV DNA levels at 24 weeks were more likely to undergo e-antigen seroconversion, achieve undetectable levels of HBV DNA, normalize ALT, and were less likely to develop resistance at one and two years.
NV-02B-015 Trial
The efficacy results of the 007 GLOBE trial were supported by results of trial NV-02B-015. This was a Phase III, randomized, double-blind, trial of Tyzeka 600 mg once daily compared to lamivudine 100 mg once daily for a treatment period of 104 weeks in 332 (n=167 Tyzeka; n=165 lamivudine) nucleoside-naïve chronic hepatitis B HBeAg-positive and HBeAg-negative Chinese subjects. The primary efficacy endpoint was serum HBV DNA reduction from baseline. In this trial, the composite endpoint Therapeutic Response was a key secondary endpoint. Histological response was not assessed as an outcome measure in this trial.
Clinical Results
Among HBeAg-positive subjects (n=147 Tyzeka; n=143 lamivudine) results for key endpoints at Week 104 included Therapeutic Response (66% vs. 41%), mean HBV DNA reduction (-5.47 vs. -3.97 log10 copies per mL), HBV DNA PCR negativity (58% vs. 34%), ALT normalization (73% vs. 59%), HBeAg loss (40% vs. 28%) and HBeAg seroconversion (29% vs. 20%), for Tyzeka and lamivudine, respectively. Because the number of HBeAg-negative subjects in this trial was small (n=42), definitive conclusions could not be drawn regarding efficacy outcomes in this subpopulation.
16. How is Tyzeka supplied
Tablets
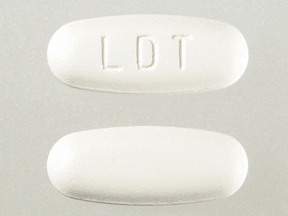
Tyzeka 600 mg tablets are white to slightly yellowish film-coated, ovaloid-shaped tablets, imprinted with “LDT” on one side.
Bottle of 30 tablets (NDC 0078-0538-15) with child-resistant closure.
Store Tyzeka tablets in original container at 25ºC (77ºF), excursions permitted to 15-30ºC (59-86ºF) [see USP Controlled Room Temperature].
Oral Solution
Tyzeka (telbivudine) oral solution is a clear, colorless to pale yellow, passion fruit flavored liquid. Tyzeka oral solution contains 100 mg of telbivudine per 5 milliliters.
Bottle containing 300 mL oral solution (NDC 0078-0539-85) with child-resistant closure and embossed dosing cup. The dosing cup is intended for measurement of Tyzeka oral solution only.
Store Tyzeka oral solution in original container at 25ºC (77ºF), excursions permitted to 15-30ºC (59-86ºF) [see USP Controlled Room Temperature]. Use within two months after opening the bottle. Do not freeze.
For all medical inquiries call: 1-877-8-Tyzeka (1-877-889-9352).
Keep this and all drugs out of the reach of children.
17. Patient Counseling Information
- See FDA-approved patient labeling (Medication Guide and Instructions for Use)
Patients should remain under the care of a physician while taking Tyzeka. They should discuss any new symptoms or concurrent medications with their physician.
Patients should be advised to report promptly unexplained muscle weakness, tenderness, or pain.
Patients should be advised to report promptly any numbness, tingling, and/or burning sensations in the arms and/or legs, with or without difficulty walking.
Patients should be advised that Tyzeka is not a cure for hepatitis B, that the long-term treatment benefits of Tyzeka are unknown at this time. In particular, the relationship of initial treatment response to outcomes such as hepatocellular carcinoma and decompensated cirrhosis is unknown.
Patients should be informed that deterioration of liver disease may occur in some cases if treatment is discontinued, and that they should discuss any change in regimen with their physician.
Patients should be advised that treatment with Tyzeka has not been shown to reduce the risk of transmission of HBV to others through sexual contact or blood contamination. HBV prevention strategies should be discussed with patients, including safe sexual practices, and avoidance of needle sharing or sharing any personal items which may contain residual blood or body fluids, such as razor blades or toothbrushes. Additionally, a vaccine is available for prevention of hepatitis B infection in susceptible individuals.
Patients on a low sodium diet should be advised that Tyzeka oral solution contains approximately 47 mg of sodium per 600 mg dose (30 mL).
Patients should be advised to dispose of unused or expired Tyzeka by using a community pharmaceutical take-back disposal program, or by placing unused Tyzeka in a closed container, such as a sealed bag, into household trash. All identifying information should be removed from the original Tyzeka container prior to disposal.
T2013-02
January 2013
Medication Guide
MEDICATION GUIDE
Tyzeka® (Tie-zee′-ka)
(telbivudine)
Tablets
Tyzeka
(telbivudine)
Oral Solution
Read this Medication Guide carefully before you start taking Tyzeka and each time you refill your prescription. There may be new information. The information contained in this Medication Guide does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is the most important information I should know about Tyzeka?
Tyzeka can cause serious side effects, including:
Lactic Acidosis (build-up of an acid in the blood). Lactic acidosis can occur in people who take medicines like Tyzeka (a nucleoside analogue). Lactic acidosis is a serious medical emergency that can lead to death. Lactic acidosis must be treated in the hospital. Women, and people who are obese, or who have taken nucleoside analogues like Tyzeka for long periods of time may be at higher risk for lactic acidosis.
Lactic acidosis can be hard to identify early, because the symptoms could seem like symptoms of other health problems. Call your healthcare provider right away if you get any of the following symptoms which could be signs of lactic acidosis:
- You feel very weak or tired.
- You have unusual (not normal) muscle pain.
- You have trouble breathing.
- You have stomach pain with nausea and vomiting.
- You feel cold, especially in your arms and legs.
- You feel dizzy or light-headed.
- You have a fast or irregular heartbeat.
Liver problems. Serious liver problems have occurred in some people who take medicines like Tyzeka. This includes liver enlargement (hepatomegaly) and fat in the liver (steatosis).
Call your healthcare provider right away if you get any of these signs of liver problems:
- Your skin or the white part of your eyes turns yellow (jaundice).
- Your urine turns dark.
- Your bowel movements (stools) turn light in color.
- You do not feel like eating food for several days or longer.
- You feel sick to your stomach (nausea).
- You have lower stomach pain.
Muscle problems (myopathy). Tyzeka can cause muscle problems, including unexplained muscle pain, muscle weakness or muscle tenderness. Serious muscle problems can occur, including muscle breakdown (rhabdomyolysis). Muscle breakdown can lead to kidney damage. Tell your healthcare provider right away if you have unexplained muscle aches, pain, tenderness, or weakness.
Nerve problems. People who take Tyzeka alone or with any type of injectable interferon product can have nerve problems such as continuing numbness, tingling, burning sensations in the arms or legs (peripheral neuropathy), or problems walking. Call your healthcare provider right away if you have any of these symptoms.
If you take Tyzeka with any type of injectable interferon product, your chance of having nerve problems may be higher and the nerve problems may be more severe. Be sure to tell your healthcare provider or pharmacist if you are also being treated with any type of injectable interferon for chronic hepatitis B or C. Check with your healthcare provider if you are not sure whether you are taking an injectable interferon product.
Worsening of your hepatitis B infection. Your hepatitis B infection may get worse or become very serious if you stop taking Tyzeka.
- Take your Tyzeka exactly as prescribed.
- Do not let your Tyzeka run out. Refill your prescription or talk to your healthcare provider before your Tyzeka is all gone.
- Do not stop taking your Tyzeka without talking to your healthcare provider.
Your healthcare provider will need to monitor your health and do regular blood tests to check your liver if you stop taking Tyzeka. Tell your healthcare provider right away about any new or unusual symptoms that happen after you stop taking Tyzeka.
What is Tyzeka?
Tyzeka is a prescription medicine used for chronic infection with hepatitis B virus (HBV) in people 16 years of age and older who also have active liver damage.
- Tyzeka will not cure HBV.
- Tyzeka may lower the amount of HBV in the body.
- Tyzeka may lower the ability of HBV to multiply and infect new liver cells.
- Tyzeka may improve the condition of your liver.
It is not known if Tyzeka is safe and effective in children younger than age 16.
Who should not take Tyzeka?
Do not take Tyzeka when you are also taking Pegasys® (pegylated interferon alfa-2a) since your chance of having nerve problems may be higher and the nerve problems may be more severe.
What should I tell my healthcare provider before I take Tyzeka?
Tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems. You may need a lower dose of Tyzeka.
- have any allergies.
- are pregnant or planning to become pregnant. It is not known if Tyzeka is safe to use during pregnancy. It is not known whether Tyzeka helps prevent a pregnant mother from passing HBV to her baby. You and your healthcare provider will need to decide if Tyzeka is right for you. If you use Tyzeka while you are pregnant, talk to your healthcare provider.
- are breast-feeding. It is not known if Tyzeka can pass into your breast milk or if it can harm your baby. Do not breast-feed if you are taking Tyzeka.
Tell your healthcare provider about all the medicines you take including prescription and nonprescription medicines, vitamins, and herbal supplements. Tyzeka may interact with other medicines that leave the body through the kidneys.
Especially tell your healthcare provider or pharmacist if you are also being treated with Pegasys® (pegylated interferon alfa-2a), or any type of injectable interferon product for chronic hepatitis B or C. Tyzeka must not be taken with Pegasys® (pegylated interferon alfa-2a) (See “What is the most important information I should know about Tyzeka?” and “Who should not take Tyzeka?”)
Tyzeka Oral Solution contains sodium. If you are on a low sodium diet, ask your healthcare provider for advice. Know the medicines you take. Keep a list of your medicines with you to show your healthcare provider and pharmacist.
How should I take Tyzeka?
Tyzeka does not stop you from spreading HBV to others by sex, sharing needles, or being exposed to your blood. Talk with your healthcare provider about safe sexual practices that protect your partner. Never share needles. Do not share personal items that can have blood or body fluids on them, like toothbrushes or razor blades. A shot (vaccine) is available to protect people at risk from becoming infected with HBV, such as partners of patients with HBV.
- Take Tyzeka exactly as prescribed. Your healthcare provider will tell you how much Tyzeka to take. The usual dose of Tyzeka is 600 mg one time each day. Your dose may be lower if you have kidney problems.
- Tyzeka may be taken with or without food.
- To help you remember to take your Tyzeka, try to take it at the same time each day.
- Do not change your dose or stop taking Tyzeka without talking to your healthcare provider first. See “What is the most important information I should know about Tyzeka?”
- Tell your healthcare provider if you have trouble swallowing tablets. Tyzeka also comes as a liquid (oral solution) that you can drink. See the end of this Medication Guide for detailed Patient Instructions for Use for the right way to take Tyzeka oral solution.
- If you forget to take Tyzeka, take it as soon as you remember and then take your next dose at the regular time. If it is almost time for your next dose, skip the missed dose. Do not take two doses at the same time. Call your healthcare provider or pharmacist if you are not sure what to do.
- If you take more than your prescribed dose of Tyzeka, call your healthcare provider right away.
- Do not change your dose or stop taking Tyzeka without talking to your healthcare provider first. See “What is the most important information I should know about Tyzeka?”
It is important to stay under your healthcare provider's care while taking Tyzeka. Your healthcare provider will regularly test the level of the hepatitis B virus in your blood.
What are the possible side effects of Tyzeka?
Tyzeka can cause serious side effects. (See "What is the most important information I should know about Tyzeka?").
Common side effects of Tyzeka include:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| |
|
Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all the possible side effects of Tyzeka. Your healthcare provider or pharmacist can give you a more complete list.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Tyzeka?
- Store Tyzeka Tablets and Oral Solution in the original bottle at room temperature, 59°F to 86°F (15° to 30°C).
- Do not store Tyzeka Tablets in a damp place such as a bathroom medicine cabinet or near the kitchen sink.
- Do not freeze Tyzeka Oral Solution.
- Use Tyzeka Oral Solution within 2 months after opening the bottle.
- Keep the bottle closed tightly.
- Throw away Tyzeka when it is outdated or no longer needed by taking Tyzeka to a community take-back disposal program, if available, or by placing Tyzeka in a closed container (such as a sealed bag) in the household trash. Remove all identifying information from the original Tyzeka container before throwing it out.
Keep Tyzeka and all medicines out of the reach of children.
General information about Tyzeka
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Tyzeka for a condition for which it was not prescribed. Do not give Tyzeka to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about Tyzeka. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Tyzeka that is written for healthcare professionals. For more information call 1-877-889-9352 or go to www.Tyzeka.com.
What are the ingredients in Tyzeka?
Active Ingredient: telbivudine
Inactive Ingredients in Tyzeka Tablets: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate. The tablet coating contains titanium dioxide, polyethylene glycol, talc, and hypromellose.
Inactive Ingredients in Tyzeka Oral Solution: citric acid anhydrous, benzoic acid, passion fruit flavor, sodium saccharin, sodium hydroxide, and purified water. A 600 mg dose (30 mL) of Tyzeka oral solution contains about 47 mg of sodium.
T2013-03
January 2013
Instructions for Use
Make sure that you read, understand, and follow these instructions carefully so that you take Tyzeka oral solution the right way.
| 1. Supplies needed to take a dose of Tyzeka oral solution | |||
 Figure 1 | To take your dose of Tyzeka you will need:
|
||
2. Important Information
|
|||
| 3. Prepare a dose of Tyzeka oral solution using the dosing cup | |||
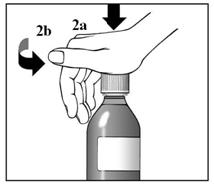 Figure 2a and 2b | 1. Remove the dosing cup from the cap. 2. Press down and turn the child-resistant cap at the same time, to open the bottle. See Figure 2a and 2b. |
||
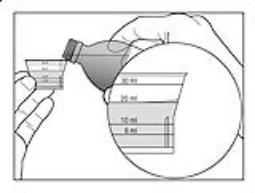 Figure 3 | 3. Before you pour the solution, check for the marking on the dosing cup that matches the amount of medicine that has been prescribed. 4. Hold the dosing cup at eye level. This will help to make sure that you measure the right amount of medicine. Carefully and slowly pour the prescribed amount of solution from the bottle into the dosing cup, until the solution reaches the line that matches the correct line on the dosing cup. See Figure 3. If you pour too much medicine into the cup, pour the extra medicine into the sink. Do not pour it back into the bottle. |
||
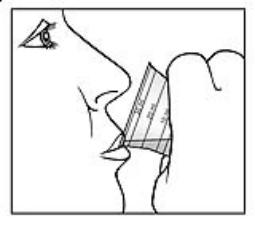 Figure 4 | 5. Take the medicine right away. See Figure 4. 6. Close the bottle tightly. |
||
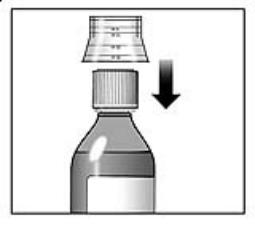 Figure 5 | 7. Rinse the dosing cup with clean water. 8. Dry the dosing cup using a dry, clean tissue before you put it back onto the cap. See Figure 5. |
||
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
This Medication Guide has been approved by the U.S. Food and Drug Administration
Tyzeka® is a registered trademark of Novartis AG.
© Novartis
T2013-08
January 2013
| TYZEKA
telbivudine tablet, film coated |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Novartis Pharmaceuticals Corporation (002147023) |
More about Tyzeka (telbivudine)
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: nucleoside reverse transcriptase inhibitors (NRTIs)
- Breastfeeding