Leqselvi: Package Insert / Prescribing Info
Package insert / product label
Generic name: deuruxolitinib phosphate
Dosage form: tablet, film coated (
Drug class: Multikinase inhibitors
Medically reviewed by Drugs.com. Last updated on Jul 27, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
LEQSELVI™ (deuruxolitinib) tablets, for oral use
Initial U.S. Approval: 2024
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE) and THROMBOSIS
See full prescribing information for complete boxed warning.
- Increased risk of serious bacterial, fungal, viral and opportunistic infections including tuberculosis (TB), that may lead to hospitalization or death. Interrupt treatment with LEQSELVI if a serious infection occurs until the infection is controlled. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test. (5.1)
- Higher rate of all-cause mortality, including sudden cardiovascular death with another Janus kinase inhibitor (JAK) vs. TNF blockers in rheumatoid arthritis (RA) patients. LEQSELVI is not approved for use in RA patients. (5.2)
- Malignancies have occurred in patients treated with LEQSELVI. Higher rate of lymphomas and lung cancers with another JAK inhibitor vs. TNF blockers in RA patients. (5.3)
- Higher rate of MACE (defined as cardiovascular death, myocardial infarction, and stroke) with another Janus kinase inhibitor (JAK) vs. TNF blockers in rheumatoid arthritis (RA) patients. (5.4)
- Thrombosis has occurred in patients treated with LEQSELVI. Increased incidence of pulmonary embolism, venous and arterial thrombosis with another JAK inhibitor vs. TNF blockers. (5.5)
Indications and Usage for Leqselvi
Leqselvi Dosage and Administration
Dosage Forms and Strengths
Tablets: 8 mg. (3)
Contraindications
Warnings and Precautions
- Increased Risk of LEQSELVI-Associated Serious Adverse Reactions in CYP2C9 Poor Metabolizers or with Concomitant Use of Moderate or Strong CYP2C9 Inhibitors: LEQSELVI is contraindicated in patients who are CYP2C9 poor metabolizers or patients taking a moderate or strong CYP2C9 inhibitor. (5.6)
- Gastrointestinal Perforations: Monitor patients who may be at increased risk for gastrointestinal perforation. Evaluate promptly patients presenting with new onset abdominal symptoms. (5.7)
- Lipid Elevations, Anemia, Neutropenia, and Lymphopenia: Monitor for changes in lipids, hemoglobin, neutrophils, and lymphocytes. (5.8)
- Immunizations: Avoid use of live vaccines during or immediately prior to LEQSELVI treatment. (5.9)
Adverse Reactions/Side Effects
Most common adverse reactions (≥1%) are: headache, acne, nasopharyngitis, blood creatine phosphokinase increased, hyperlipidemia, fatigue, weight increased, lymphopenia, thrombocytosis, anemia, skin and soft tissue infections, neutropenia, and herpes. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sun Pharmaceutical Industries, Inc. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Use In Specific Populations
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2024
Full Prescribing Information
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, AND THROMBOSIS
- Increased risk of serious bacterial, fungal, viral and opportunistic infections, including tuberculosis (TB), that may lead to hospitalization or death. Interrupt treatment with LEQSELVI if a serious infection occurs until the infection is controlled. LEQSELVI treatment is not recommended in patients with active tuberculosis. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test. (5.1)
- Higher rate of all-cause mortality, including sudden cardiovascular death, was observed with another Janus kinase (JAK) inhibitor vs. TNF blockers in rheumatoid arthritis (RA) patients. LEQSELVI is not approved for use in RA patients. (5.2)
- Malignancies were reported in patients treated with LEQSELVI. Higher rate of lymphomas and lung cancers was observed with another JAK inhibitor vs. TNF blockers in RA patients. (5.3)
- Higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke) was observed with another JAK inhibitor vs. TNF blockers in RA patients. (5.4)
- Thrombosis, including cerebral venous sinus thrombosis (CVT), deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients treated with LEQSELVI. Increased incidence of pulmonary embolism, venous and arterial thrombosis was observed with another JAK inhibitor vs. TNF blockers. (5.5)
1. Indications and Usage for Leqselvi
LEQSELVI™ is indicated for the treatment of adult patients with severe alopecia areata.
Limitations of Use
LEQSELVI is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, cyclosporine, or other potent immunosuppressants.
2. Leqselvi Dosage and Administration
2.1 Recommended Evaluations and Immunizations Prior to and During Treatment
Perform the following prior to treatment with LEQSELVI:
- CYP2C9 genotype determination: Test patients for CYP2C9 variants to determine CYP2C9 genotype. LEQSELVI is contraindicated in patients who are CYP2C9 poor metabolizers (patients with decreased cytochrome P450 (CYP) 2C9 function) [see Contraindications (4)]. An FDA-cleared or -approved test for the detection of CYP2C9 variants to direct the use of LEQSELVI is not currently available.
- Evaluation for use of concomitant CYP2C9 inhibitors: LEQSELVI is contraindicated in patients taking moderate or strong CYP2C9 inhibitors [see Warnings and Precautions (5.6)].
- Active and latent tuberculosis (TB) evaluation: LEQSELVI treatment is not recommended in patients with active TB. For patients with latent TB or those with a negative latent TB test who are at high risk of TB, start preventive therapy for TB prior to LEQSELVI treatment [see Warnings and Precautions (5.1)].
- Viral hepatitis screening in accordance with clinical guidelines: LEQSELVI treatment is not recommended in patients with active hepatitis B or hepatitis C.
- Hepatitis B infection screening: If hepatitis B infection is discovered, follow hepatitis B clinical guidelines, or refer to a liver specialist. Monitor patients for reactivation in accordance with clinical guidelines during treatment [see Warnings and Precautions (5.1)].
- Complete blood count (CBC): LEQSELVI treatment is not recommended in patients with an absolute lymphocyte count (ALC) <500 cells/mm3 absolute neutrophil count (ANC) <1,000 cells/mm3, or hemoglobin level <8 g/dl. Monitor complete blood counts periodically during treatment and modify dosage as recommended [see Dosage and Administration (2.3) and Warnings and Precautions (5.8)].
- Complete any necessary immunizations, including herpes zoster vaccinations, according to current immunization guidelines prior to LEQSELVI treatment [see Warnings and Precautions (5.9)].
2.2 Recommended Dosage
The recommended dosage of LEQSELVI for the treatment of severe alopecia areata is 8 mg orally twice daily, with or without food [see Clinical Pharmacology (12.3)].
If a dose is missed, skip the missed dose and resume dosing at the next scheduled dose.
2.3 Treatment Interruption and Resumption
Serious or Opportunistic Infections
If a patient develops a serious or opportunistic infection, interrupt LEQSELVI treatment until the infection is controlled [see Warnings and Precautions (5.1)].
Hematological Abnormalities
Recommendations for LEQSELVI treatment interruption for hematologic abnormalities are described in Table 1 [see Warnings and Precautions (5.8)].
| Laboratory Measure | Interruption Criterion | Resumption Criterion |
| Absolute Lymphocyte Count (ALC) | <500 cells/mm3 | ≥500 cells/mm3 |
| Absolute Neutrophil Count (ANC) | <1000 cells/mm3 | ≥1000 cells/mm3 |
| Hemoglobin | <8 g/dL | ≥8 g/dL |
3. Dosage Forms and Strengths
Tablet: purple, round, debossed with “C” on one side and “8” on the other side
5. Warnings and Precautions
5.1 Serious Infections
Serious infections have been reported in subjects with alopecia areata receiving LEQSELVI [see Adverse Reactions (6.1)].
Avoid use of LEQSELVI in patients with an active, serious infection including localized infections.
Prior to LEQSELVI treatment, consider the risks and benefits in patients:
- with chronic or recurrent infection
- who have been exposed to tuberculosis
- with a history of a serious or opportunistic infection
- who have resided or traveled in areas of endemic tuberculosis or endemic mycoses; or
- with underlying conditions that may predispose them to infection
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with LEQSELVI. If the patient develops a serious infection, interrupt treatment with LEQSELVI until the infection resolves or is adequately treated. If a patient develops a new infection during treatment with LEQSELVI, initiate complete diagnostic testing appropriate for an immunocompromised patient and appropriate antimicrobial therapy.
Tuberculosis
Evaluate patients for latent and active tuberculosis (TB) infection prior to LEQSELVI treatment. LEQSELVI is not recommended for use in patients with active TB.
Treat patients with latent TB before LEQSELVI treatment. Consider anti-TB therapy prior to LEQSELVI treatment in patients with previously untreated latent TB or active TB in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent TB but who have risk factors for TB infection. Consultation with a physician with expertise in the treatment of TB is recommended to aid in the decision about whether initiating anti-TB therapy is appropriate for an individual patient.
Monitor patients receiving LEQSELVI for signs and symptoms of active TB during treatment, including patients who tested negative for latent TB infection prior to treatment.
Viral Reactivation
Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster) were reported in clinical trials with LEQSELVI [see Adverse Reactions (6.1)]. If a patient develops herpes zoster, consider interrupting LEQSELVI treatment until the episode resolves.
The impact of LEQSELVI on chronic viral hepatitis reactivation is unknown. Subjects with positive results for hepatitis B surface antigens (HBsAg), antibodies to hepatitis B core antigens (anti-HBc), or hepatitis C virus (HCV) with detectable HCV RNA at screening were excluded from LEQSELVI clinical trials. Perform screening for viral hepatitis before treatment with LEQSELVI. LEQSELVI is not recommended for use in patients with active hepatitis B or hepatitis C (HCV RNA detected).
If non-active hepatitis B infection is discovered, monitoring for reactivation or prophylactic treatment is recommended. Follow hepatitis B clinical guidelines or refer to a liver specialist. Hepatitis B viral load (HBV-DNA titer) increase, with or without associated elevations in alanine aminotransferase and aspartate aminotransferase, has been reported in subjects with chronic HBV infections receiving JAK inhibitors used to treat inflammatory conditions. The effect of LEQSELVI on viral replication in patients with chronic HBV infection is unknown.
5.2 Mortality
In a large, randomized, postmarketing safety trial of another JAK inhibitor in rheumatoid arthritis (RA) subjects 50 years and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in subjects treated with the JAK inhibitor compared with TNF blockers.
Consider the benefits and risks for the individual patient prior to and during treatment with LEQSELVI.
5.3 Malignancy and Lymphoproliferative Disorders
Malignancies were observed in clinical trials of LEQSELVI [see Adverse Reactions 6.1)].
In a large, randomized, postmarketing safety trial of another JAK inhibitor in subjects with RA, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed in subjects treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in subjects treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this trial, current or past smokers had an additional increased risk of overall malignancies.
Consider the benefits and risks for the individual patient prior to and during treatment with LEQSELVI, particularly in patients with a known malignancy (other than successfully treated NMSC), patients who develop a malignancy, and patients who are current or past smokers.
Non-melanoma skin cancers
Non-melanoma skin cancers (NMSCs) have been reported in patients treated with LEQSELVI. Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
5.4 Major Adverse Cardiovascular Events (MACE)
In a large, randomized, postmarketing safety trial of another JAK inhibitor in subjects with RA 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to and during treatment with LEQSELVI, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Inform patients about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue LEQSELVI in patients that have experienced a myocardial infarction or stroke.
5.5 Thrombosis
Thrombosis, including pulmonary embolism (PE), deep vein thrombosis (DVT) and cerebral venous sinus thrombosis (CVT) have been reported in clinical trials of deuruxolitinib [see Adverse Reactions (6.1)]. There was no clear relationship between platelet count elevations and thrombotic events.
Thrombosis, including DVT, PE, and arterial thrombosis have been reported in subjects receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death.
In a large, randomized, postmarketing safety trial of another JAK inhibitor in subjects with RA 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers.
Avoid LEQSELVI in patients who may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue LEQSELVI and evaluate and treat patients appropriately.
5.6 Increased Risk of LEQSELVI-Associated Serious Adverse Reactions in CYP2C9 Poor Metabolizers or with Concomitant Use of Moderate or Strong CYP2C9 Inhibitors
Higher plasma concentrations of deuruxolitinib, which may increase the risk of LEQSELVI-associated serious adverse reactions such as thrombosis, may occur when LEQSELVI is used in patients who:
- Are CYP2C9 poor metabolizers [see Use in Specific Populations (8.8) and Clinical Pharmacology (12.5)].
- Are on a concomitant moderate or strong CYP2C9 inhibitor [see Drug Interactions (7) and Clinical Pharmacology (12.3)].
Prior to LEQSELVI treatment, test patients for CYP2C9 variants to determine if they are poor metabolizers [see Dosage and Administration (2.1)]. An FDA-cleared or -approved test for the detection of CYP2C9 variants to direct the use of LEQSELVI is not currently available.
LEQSELVI is contraindicated in patients who are CYP2C9 poor metabolizers or patients who are on concomitant moderate or strong CYP2C9 inhibitors [see Contraindications (4)].
5.7 Gastrointestinal Perforations
Gastrointestinal perforations have been reported in clinical trials with LEQSELVI.
Monitor patients treated with LEQSELVI who may be at increased risk for gastrointestinal perforation (e.g., patients with a history of diverticulitis). Evaluate promptly patients presenting with new onset abdominal symptoms for early identification of gastrointestinal perforation.
5.8 Lipid Elevations, Anemia, Neutropenia, and Lymphopenia
Perform a CBC prior to and periodically during treatment with LEQSELVI [see Dosage and Administration (2.1)].
Lipid Elevations
Treatment with LEQSELVI was associated with increases in triglycerides and total cholesterol, including HDL-C and LDL-C [see Adverse Reactions 6.1)]. The effect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined. Perform assessment of lipid parameters at baseline and periodically during treatment with LEQSELVI. Manage patients according to clinical guidelines for hyperlipidemia.
Anemia
Treatment with LEQSELVI was associated with an increased incidence of anemia (hemoglobin less than 8 g/dL) compared to placebo [see Adverse Reactions (6.1)]. Avoid or interrupt LEQSELVI treatment in patients with hemoglobin less than 8 g/dL [see Dosage and Administration (2.3)].
Neutropenia
Treatment with LEQSELVI was associated with an increased incidence of neutropenia (ANC less than 1000 cells/mm3) compared to placebo [see Adverse Reactions (6.1)]. Avoid or interrupt LEQSELVI treatment in patients with an ANC less than 1,000 cells/mm3[see Dosage and Administration (2.3)].
Lymphopenia
Treatment with LEQSELVI was associated with an increased incidence of lymphopenia (ALC less than 500 cells/mm3) compared to placebo [see Adverse Reactions (6.1)]. Avoid or interrupt LEQSELVI treatment in patients with an ALC less than 500 cells/mm3[see Dosage and Administration (2.3)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Serious Infections [see Warnings and Precautions (5.1)]
- Malignancy and Lymphoproliferative Disorders [see Warnings and Precautions (5.3)]
- Thrombosis [see Warnings and Precautions (5.5)]
- Gastrointestinal perforations [see Warnings and Precautions (5.7)]
- Lipid Elevations, Anemia, Neutropenia and Lymphopenia [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of LEQSELVI was evaluated in three randomized, placebo-controlled clinical trials (including a dose-ranging trial), two open-label trials, and two long-term extension trials in adult subjects with severe alopecia areata. These subjects had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT) for more than six months. A total of 1,730 subjects with alopecia areata were treated across all trials, representing 1,962.9 patient-years of exposure. There were 974 subjects who were exposed to either LEQSELVI 8 mg or deuruxolitinib 12 mg for at least 1 year and 104 subjects who were exposed for at least 3 years.
Deuruxolitinib 12 mg is not approved.
Among 1,020 subjects enrolled in the placebo-controlled clinical trials, 640 subjects received LEQSELVI 8 mg twice daily, 380 subjects received deuruxolitinib 12 mg twice daily and 299 subjects received placebo twice daily for up to 24 weeks [see Clinical Studies (14)].
Adverse Reactions occurring at ≥1% in the LEQSELVI 8 mg or deuruxolitinib 12 mg twice daily group and at a higher rate than in the placebo group are presented in Table 2. A total of 20 (3.1%) of subjects treated with LEQSELVI 8 mg were discontinued from the trials due to adverse reactions.
| a. %-study size adjusted percentages. b. Acne includes: acne, dermatitis acneiform, and acne pustular. c. Hyperlipidemia includes: blood cholesterol increased, low density lipoprotein increased, blood triglycerides increased, hypercholesterolemia, hyperlipidemia, hypertriglyceridemia, and dyslipidaemia. d. Fatigue includes: fatigue, asthenia, hypersomnia, somnolence, and lethargy. e. Skin and soft issue infections includes: folliculitis, impetigo, skin infection, subcutaneous abscess, furuncle, paronychia, and pustule. f. Anemia includes: anemia, hematocrit decreased, hemoglobin decreased, iron deficiency anemia, and red blood cell count decreased. g. Neutropenia includes: neutropenia and neutrophil count decreased. h. Thrombocytosis includes: thrombocytosis and platelet count increased. i. Herpes includes: oral herpes, herpes simplex, genital herpes simplex, and nasal herpes. |
|||
| Placebo N = 299 n (%)a | LEQSELVI 8 mg twice daily N = 640 n (%)a | Deuruxolitinib 12 mg twice daily N = 380 n (%)a |
|
| Acneb | 13 (4.3) | 66 (10.0) | 52 (12.6) |
| Headache | 30 (9.4) | 83 (12.4) | 44 (10.5) |
| Nasopharyngitis | 21 (6.7) | 54 (8.1) | 33 (7.7) |
| Blood creatine phosphokinase increased | 7 (2.2) | 35 (5.3) | 27 (7.4) |
| Hyperlipidemiac | 10 (3.1) | 30 (4.4) | 19 (5.2) |
| Fatigued | 12 (3.9) | 26 (3.9) | 20 (4.9) |
| Skin and soft tissue infectionse | 2 (0.8) | 11 (1.6) | 15 (4.0) |
| Anemiaf | 3 (1.0) | 18 (2.6) | 16 (3.4) |
| Weight increased | 4 (1.4) | 19 (2.9) | 10 (2.5) |
| Neutropeniag | 3 (0.7) | 10 (1.4) | 10 (2.8) |
| Lymphopenia | 2 (0.6) | 2 (0.3) | 7 (2.0) |
| Thrombocytosish | 0 | 18 (2.7) | 6 (1.6) |
| Herpesi | 2 (0.6) | 8 (1.2) | 6 (1.6) |
Additional adverse drug reactions occurring in fewer than 1% of subjects: herpes zoster, lipase increased, and candidiasis.
A total of 868 subjects in the long-term extension trials received treatment with LEQSELVI 8 mg twice daily and 991 subjects received treatment with deuruxolitinib 12 mg twice daily for 52 weeks. In two open-label extension trials up to 3 years, 829 subjects received treatment with LEQSELVI 8 mg twice daily, and 1066 subjects received treatment with deuruxolitinib 12 mg twice daily.
Specific Adverse Reactions (0-52 weeks)
All Infections
During the 24-week treatment period, infections were reported in 97 subjects (88.0 per 100 patient-years) treated with placebo, 222 subjects (101.5 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 153 subjects (117.0 per 100 patient years) treated with deuruxolitinib 12 mg twice daily. During the 0-52 week period, infections were reported in 435 subjects (95.5 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 408 subjects (74.1 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily.
Serious Infections
During the 24-week treatment period, serious infections were reported in 1 subject (0.8 per 100 patient-years) treated with placebo, 5 subjects (1.8 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 2 subjects (1.2 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily. During the 0-52 week period, serious infections were reported in 5 subjects (0.7 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 4 subjects (0.5 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily.
Herpes Zoster
During the 24-week treatment period, opportunistic infections (herpes zoster) were reported in 0 subjects treated with placebo, 3 subjects (1.1 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 3 subjects (1.8 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily. During the 0-52 week period, herpes zoster was reported in 10 subjects (1.5 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 15 subjects (1.9 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily.
Malignancies
During the 0-52 week period, malignancy excluding NMSC was reported in 3 subjects (0.4 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 4 subjects (0.5 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily.
Thrombosis
During the 0-52 week period, thrombosis was reported in 0 subjects treated with LEQSELVI 8 mg twice daily and 1 subject (0.1 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily who developed bilateral pulmonary embolism.
Laboratory Abnormalities
Anemia
During the 24-week treatment period, anemia was reported in 3 subjects (2.3 per 100 patient-years) treated with placebo, 19 subjects (6.9 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 16 subjects (9.6 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily. During the 0-52 week period, anemia was reported in 17 subjects (2.6 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 38 subjects (5.0 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily.
Neutropenia
During the 24-week treatment period, neutropenia was reported in 3 subjects (2.3 per 100 patient-years) treated with placebo, 10 subjects (3.6 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 10 subjects (6.0 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily. During the 0-52 week period, neutropenia was reported in 11 subjects (1.6 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 15 subjects (1.9 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily.
Lymphopenia
During the 24-week treatment period, lymphopenia was reported in 2 subjects (1.5 per 100 patient-years) treated with placebo, 2 subjects (0.7 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 7 subjects (4.2 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily. During the 0-52 week period, lymphopenia was reported in 4 subjects (0.6 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 10 subjects (1.3 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily.
Lipid Elevations
During the 24-week treatment period, lipid elevations were reported in 10 subjects (7.7 per 100 patient-years) treated with placebo, 30 subjects (11.0 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 18 subjects (10.9 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily. During the 0-52 week period, lipid elevations were reported in 47 subjects (7.2 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 66 subjects (8.7 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily.
Creatine Phosphokinase (CPK) Elevations
During the 24-week treatment period, CPK elevations were reported in 7 subjects (5.4 per 100 patient-years) treated with placebo, 35 subjects (12.9 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 27 subjects (16.6 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily. During the 0-52 week period, CPK elevations were reported in 49 subjects (7.6 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 46 subjects (6.1 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily.
Thrombocytosis
During the 24-week treatment period, an increase in platelet count was reported in 0 subjects treated with placebo, 18 subjects (6.6 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 6 subjects (3.6 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily.
During the 0-52 week period, an increase in platelet count was reported in 20 subjects (3.0 per 100 patient-years) treated with LEQSELVI 8 mg twice daily and 26 subjects (3.4 per 100 patient-years) treated with deuruxolitinib 12 mg twice daily.
Adverse Reactions Observed after 52 weeks
Thrombosis
Venous thromboembolic events were reported in 4 subjects treated with deuruxolitinib 12 mg twice daily between Week 52 and Week 98. These 4 subjects experienced 7 thrombotic events (0.2 per 100 patient-years), including deep vein thrombosis (DVT), bilateral pulmonary embolism (PE), pulmonary embolism, and cerebral venous sinus thrombosis (CVT).
Related/similar drugs
7. Drug Interactions
Effect of Other Drugs on LEQSELVI
Strong CYP3A and moderate or strong CYP2C9 inducers:
Avoid concomitant use of LEQSELVI with strong CYP3A and moderate or strong CYP2C9 inducers.
Deuruxolitinib is a CYP2C9 and CYP3A substrate. Concomitant use with a strong CYP3A and moderate or strong CYP2C9 inducer decreases deuruxolitinib exposure (Cmax and AUC), which may reduce LEQSELVI efficacy [see Clinical Pharmacology (12.3)].
Moderate or strong CYP2C9 inhibitors:
LEQSELVI is contraindicated in patients taking moderate or strong CYP2C9 inhibitors [see Contraindications (4)].
Deuruxolitinib is a CYP2C9 substrate. Concomitant use with a moderate or strong CYP2C9 inhibitor is estimated to increase deuruxolitinib exposure (Cmax and AUC), which may increase the risk of LEQSELVI serious adverse reactions such as thrombosis [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on the findings from animal reproduction studies, deuruxolitinib may cause fetal harm during pregnancy. Available data from pregnancies reported in clinical trials with LEQSELVI are not sufficient to evaluate for a drug-associated risk for major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of deuruxolitinib to pregnant rats during the period of organogenesis at a dose 4.8 times the maximum recommended human dose (MRHD) resulted in reduced fetal weight and increased skeletal malformation. Oral administration of deuruxolitinib to pregnant rabbits during the period of organogenesis at a dose 0.3 times the MRHD resulted in maternal toxicity, reduced fetal weight, and increased post-implantation loss. Oral administration of deuruxolitinib to pregnant rats during pregnancy and lactation periods at a dose 5 times the MRHD resulted in maternal toxicity, decreased pup survival, and adverse effects on postnatal development (see Data). Advise pregnant women of the potential risk to a fetus.
The background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects is 2 to 4% of the general population and miscarriage occurs in 15 to 20% of clinically recognized pregnancies.
Animal Data
In an embryo-fetal development study, deuruxolitinib was administered to pregnant rats during the period of organogenesis at oral doses of 15, 30, and 60 mg/kg/day. Reduced fetal weight and increased fetal skeletal malformation were noted at 60 mg/kg/day (4.8 times the MRHD based on AUC comparison) with no maternal toxicity. No embryo-fetal toxicity was observed at doses up to 30 mg/kg/day (equivalent to MRHD based on AUC comparison). In another embryo-fetal development study, deuruxolitinib was administered to pregnant rabbits during the period of organogenesis at oral doses of 6, 30, and 60 mg/kg/day. Reduced fetal weight and increased post-implantation loss were noted at 60 mg/kg/day (0.3 times the MRHD based on AUC comparison), at which maternal toxicity was observed. No embryo-fetal toxicity was noted at doses up to 30 mg/kg/day (0.05 times the MRHD based on AUC comparison).
In a pre- and postnatal development study in rats, deuruxolitinib was administered to pregnant rats during pregnancy and lactation periods at oral doses of 15, 30, and 75 mg/kg/day. Decreases in liveborn pups and pup survival, decreased pup activity and lower pup body weights, and adverse effects on reproductive outcome in the second generation females (decreased corpora lutea, implantation, and number of live embryos, and increased resorption and post-implantation loss) were noted at 75 mg/kg/day (5 times the MRHD based on AUC comparison), at which maternal toxicity was also observed. No adverse effects on pre- and postnatal development were noted at doses up to 30 mg/kg/day (0.8 times the MRHD based on AUC comparison).
8.2 Lactation
Risk Summary
There are no data on the presence of deuruxolitinib in human milk, the effects on the breast-fed infant, or the effects on milk production. Deuruxolitinib was present in the milk of lactating rats (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the potential for serious adverse reactions in nursing infants, advise women not to breastfeed during treatment with LEQSELVI and for one day after the last dose (approximately 5 to 6 elimination half-lives).
Animal Data
Following a single oral dose of 10 mg/kg administered to lactating rats on lactation day 14, deuruxolitinib concentrations were up to 20 times higher in milk than in plasma.
8.3 Females and Males of Reproductive Potential
Contraception
Females
Based on animal studies, deuruxolitinib may cause fetal harm when administered during pregnancy [see Use in Specific Populations (8.1)]. Consider pregnancy planning and prevention for females of reproductive potential.
8.4 Pediatric Use
The safety and effectiveness of LEQSELVI have not been established in pediatric patients.
8.5 Geriatric Use
Of the 600 subjects treated with LEQSELVI 8 mg in phase 3 clinical trials, 2 (0.3%) were 65 years of age or older. Clinical trials of LEQSELVI did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects.
8.6 Renal Impairment
LEQSELVI is not recommended for use in patients with severe renal impairment or end-stage renal disease (eGFR < 30 ml/min). No adjustment of dosage is required in patients with mild or moderate renal impairment.
The effect of severe renal impairment on deuruxolitinib pharmacokinetics is unknown [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
LEQSELVI is not recommended for use in patients with severe hepatic impairment (Child Pugh C). No adjustment of dosage is required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment.
The effect of severe hepatic impairment on deuruxolitinib pharmacokinetics is unknown [see Clinical Pharmacology (12.3)].
8.8 CYP2C9 Poor Metabolizers
Based on modeling, higher exposure of deuruxolitinib in patients who are CYP2C9 poor metabolizers is expected with concomitant use of LEQSELVI, which may increase the risk of LEQSELVI-associated serious adverse reactions. Before initiation of treatment with LEQSELVI, test patients to determine CYP2C9 genotype. An FDA-cleared or -approved test for the detection of CYP2C9 variants to direct the use of LEQSELVI is not currently available [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.5)].
10. Overdosage
There is no experience regarding human overdose with LEQSELVI.
There is no specific antidote for overdose with LEQSELVI. Treatment should be symptomatic and supportive and monitor patients for signs and symptoms of adverse reactions [see Adverse Reactions (6.1)].
In case of overdose, consider contacting the Poison Center at 1-800-222-1222 for latest recommendations.
11. Leqselvi Description
LEQSELVI (deuruxolitinib) tablets contain the phosphate salt of deuruxolitinib, a Janus kinase (JAK) inhibitor, for oral administration.
Deuruxolitinib phosphate is a white to off-white crystalline solid with the chemical name 1H-Pyrazole-1-propanenitrile, β-(cyclopentyl-2,2,3,3,4,4,5,5-d8)-4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-, (βR)-, phosphate (1:1).
Deuruxolitinib has high aqueous solubility at low pH. Deuruxolitinib phosphate has a molecular weight of 412.42 g/mol and a molecular formula of C17H13D8N6O4P. The structural formula is:

Each tablet contains 8 mg of deuruxolitinib (equivalent to 10.50 mg of deuruxolitinib phosphate) and the following excipients: colloidal silicon dioxide, lactose monohydrate, low-substituted hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose and povidone. The tablet film coating contains the following excipients: carmine, FD&C blue #2 aluminum lake, glyceryl mono and dicaprylocaprate, polyvinyl alcohol, sodium lauryl sulfate, talc, and titanium dioxide.
12. Leqselvi - Clinical Pharmacology
12.1 Mechanism of Action
Deuruxolitinib is a Janus kinase (JAK) inhibitor. JAKs mediate the signaling of a number of cytokines and growth factors that are important for hematopoiesis and immune function. JAK signaling involves recruitment of STATs (signal transducers and activators of transcription) to cytokine receptors, activation and subsequent localization of STATs to the nucleus leading to modulation of gene expression.
In an in vitro kinase activity assay, deuruxolitinib had greater inhibitory potency for JAK1, JAK2 and TYK2 relative to JAK3. The relevance of inhibition of JAK enzymes to therapeutic effectiveness is not currently known.
12.2 Pharmacodynamics
Deuruxolitinib Inhibition of IL-6 Induced STAT3 Phosphorylation
Deuruxolitinib inhibited whole blood IL-6 stimulated pSTAT3 in healthy subjects 2 hours post-dose. The relevance of this finding in patients is unknown.
Cardiac Electrophysiology
At concentrations approximately 4-fold higher than the Cmax associated with the highest dose evaluated clinically, 12 mg twice-daily, deuruxolitinib does not prolong the QT interval to any clinically relevant extent.
12.3 Pharmacokinetics
Following oral administration of deuruxolitinib, Cmax and AUCs increased dose proportionally over a dose range from 8 mg to 48 mg (6 times the approved recommended dosage) in healthy subjects. Steady-state plasma concentrations were achieved within 1 to 2 days, with minimal accumulation, after twice daily administration.
Absorption
Deuruxolitinib bioavailability is 90%, with peak plasma concentrations reached within 1.5 hrs.
Effect of Food
No clinically significant differences in the pharmacokinetics of deuruxolitinib were observed following administration of a high fat, high calorie meal (approximately 50% fat and 800-1000 calories).
Distribution
The deuruxolitinib steady state volume of distribution is approximately 50L. Deuruxolitinib plasma protein binding is 91.5% and blood to plasma concentration ratio is approximately 1.3.
Elimination
The deuruxolitinib mean elimination half-life is approximately 4 hrs.
Metabolism
Deuruxolitinib is primarily metabolized by CYP2C9 (76%) and CYP3A4 (21%) and to a lesser extent by CYP1A2 (3%). The two most abundant human metabolites C-21714 and C-21717, each of which accounted for approximately 5% of total drug-related AUC and both are approximately 10-fold less pharmacologically active than deuruxolitinib.
Excretion
After a single dose of radiolabeled deuruxolitinib, there was no unchanged dose recovered in either urine or feces.
Specific Populations
No clinically significant differences in the pharmacokinetics of deuruxolitinib were observed based on race [White (75%), African American (17%) and Asian (6%)], ethnicity [Hispanic or Latino (12%)], age (18-65 years), body weight (40.4-173 kg), mild to moderate renal impairment (eGFR 30-89 mL/min, MDRD), or mild to moderate hepatic impairment (Child Pugh A or B). The effect of severe renal impairment (eGFR < 30 mL/min, MDRD) or severe hepatic impairment (Child Pugh C) on deuruxolitinib pharmacokinetics is unknown.
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Effect of Other Drugs on LEQSELVI
Strong CYP3A4 and Moderate or strong CYP2C9 Inducers: Deuruxolitinib AUC decreased by 78% and Cmax by 41% following concomitant use of multiple doses of 600 mg rifampin (strong CYP3A4 and moderate CYP2C9 inducer) with a single dose of 12 mg deuruxolitinib (1.5 times the approved 8 mg dose).
Strong CYP2C9 Inhibitors: Based on modeling, deuruxolitinib AUC is predicted to be increased by 200% and Cmax by 25% following concomitant use of multiple dosages of a strong CYP2C9 inhibitor with a single dose of 12 mg deuruxolitinib (1.5 times the approved 8 mg dose).
Moderate CYP2C9 Inhibitors: Deuruxolitinib AUC increased by 140% and Cmax by 21% following concomitant use of multiple dosages of 200 mg fluconazole (dual moderate CYP3A4 and CYP2C9 inhibitor) with a single dose of 12 mg deuruxolitinib (1.5 times the approved 8 mg dose).
Other Drugs: No clinically significant differences in deuruxolitinib pharmacokinetics were observed when used concomitantly with itraconazole (strong CYP3A4 inhibitor) or are expected with efavirenz (moderate CYP3A4 inducer).
Effect of LEQSELVI on Other Drugs:
No clinically significant differences in the pharmacokinetics of the following drugs were observed when co-administered with deuruxolitinib: midazolam (a sensitive CYP3A4 substrate), oral contraceptives (ethinyl estradiol and levonorgestrel).
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: Deuruxolitinib is not an inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C19, CYP2D6, or CYP3A4. Deuruxolitinib is not an inducer of CYP1A2 or CYP2B6, CYP2C8 or CYP2C19.
Transporter Systems: Deuruxolitinib is a substrate of BCRP and MDR1 but not a substrate of the uptake transporters OATP1B1 and OATP1B3. Deuruxolitinib is not an inhibitor of OATP1B1, OATP1B3 and OCT1 but is an inhibitor of BCRP, BSEP, OAT3 and MATE2-K.
12.5 Pharmacogenomics
Deuruxolitinib is primarily metabolized by CYP2C9 (76%) and CYP3A4 (21%). CYP2C9 activity is reduced in patients with genetic variants in CYP2C9, such as the CYP2C9∗2 and CYP2C9∗3 alleles. The impact of CYP2C9 genetic variants on the pharmacokinetics of deuruxolitinib has not been directly evaluated. Based on drug-drug interaction modeling data, CYP2C9 poor metabolizers (e.g., ∗2/∗3, ∗3/∗3) may have up to 2-fold higher concentrations of deuruxolitinib, when compared to normal metabolizers [see Warnings and Precautions (5.6)].
The pharmacokinetics of deuruxolitinib were not evaluated in individuals who are intermediate metabolizers (e.g., individuals with ∗1/∗3 genotype).
The prevalence of the CYP2C9 poor metabolizer phenotype is approximately 2 to 3% in White populations, 0.5 to 4% in Asian populations, and <1% in Black or African American populations. Other decreased or nonfunctional CYP2C9 alleles (e.g., ∗5, ∗6, ∗8, ∗11) are more prevalent in Black or African American populations.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Deuruxolitinib was not carcinogenic when administered orally in a 6-month transgenic rasH2 mouse study at doses up to 100 mg/kg/day. In a 2-year rat carcinogenicity study, no drug-related tumors were observed at oral doses of deuruxolitinib up to 30 mg/kg/day (0.6 times the MRHD based on AUC comparison).
Deuruxolitinib was positive in an in vitro micronucleus assay, but negative in a bacterial mutation assay (the Ames test), an in vitro chromosome aberration assay and an in vivo rat micronucleus assay.
In fertility and early embryonic development studies in rats, deuruxolitinib was administered to male rats prior to mating to conception, or to female rats prior to mating, through conception, to gestation day 7. Deuruxolitinib had no adverse effects on male or female fertility at oral doses up to 100 mg/kg/day (2.2 times MRHD in males and 14 times MRHD in females based on AUC comparison). However, adverse effects on early embryonic development were noted, including decreased viable embryos and increased pre-implantation loss observed at doses ≥ 30 mg/kg/day (0.9 times MRHD based on AUC comparison), and increased post-implantation loss and resorption at 100 mg/kg/day (14 times MRHD based on AUC comparison). No adverse effects on early embryonic development were observed at 10 mg/kg/day (0.2 times MRHD based on AUC comparison).
14. Clinical Studies
Two multicenter, randomized, double-blind, placebo-controlled phase 3 clinical trials (AA-1 [NCT04518995] and AA-2 [NCT04797650]), evaluated a total of 1,209 adult subjects with alopecia areata (AA), who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT) for more than six months. In both trials, subjects received LEQSELVI 8 mg twice daily, deuruxolitinib 12 mg twice daily, or placebo twice daily for 24 weeks.
Deuruxolitinib 12 mg is not approved.
The trial population ranged from 18 to 65 years of age. Among the subjects enrolled, 64% were female, 74% were White, 9% were Black or African American, and 6% were Asian; 8% identified as Hispanic or Latino. At baseline, subjects had average current episode of hair loss of approximately 4 years, with 59% of subjects having complete or near complete scalp hair loss (defined as ≥ 95% scalp hair loss). The mean pooled baseline SALT scores across treatment groups ranged from 85.9 to 88.6 with a mean duration of current episode of hair loss of ranging 3.7 to 3.9 years. Approximately 73% of subjects had eyebrow hair involvement and 70% of subjects had eyelash hair involvement.
The primary endpoint for both trials assessed the proportion of subjects who achieved at least 80% scalp hair coverage (SALT score of ≤ 20) at Week 24. Key secondary outcomes included the percentage of responders (defined as “satisfied” or “very satisfied”) at Week 24 on the Satisfaction of Hair Patient-Reported Outcome (SPRO) and the percentage of subjects achieving an absolute SALT score of ≤ 20 at Week 20, 16, 12, and 8.
Upon completion of the 24-week trials, subjects were eligible to enroll in a long-term extension trial.
Clinical Response
Assessment of scalp hair loss was based on the SALT score. At Week 24, a greater proportion of subjects had a SALT ≤ 20 response (80% or more scalp hair) and SALT ≤ 10 response (90% or more scalp hair) with LEQSELVI 8 mg twice daily compared to placebo (Table 3).
|
Trial AA-1 |
Trial AA-2 |
|||
|
a Not adjusted for multiplicity. |
||||
|
Placebo |
LEQSELVI |
Placebo |
LEQSELVI |
|
|
SALT scores ≤ 20 |
1% |
29% |
1% |
32% |
|
SALT scores ≤ 10 |
0%a |
20% |
0%a |
24% |
|
Trial AA-1 |
Trial AA-2 |
|||
|
a In Trial AA-1, the proportion of responders (defined as subjects who were “satisfied” or “very satisfied”) on LEQSELVI was 42% compared to 5% on placebo. In Trial AA-2, the proportion of responders on LEQSELVI was 46% compared to 2% on placebo. |
||||
|
Placebo |
LEQSELVI |
Placebo |
LEQSELVI |
|
|
“Very Satisfied”a |
2% |
19% |
0% |
18% |
|
“Satisfied”a |
3% |
23% |
2% |
28% |
|
“Neither Satisfied nor Dissatisfied” |
16% |
19% |
13% |
15% |
|
“Dissatisfied” |
25% |
18% |
18% |
16% |
|
“Very Dissatisfied” |
54% |
21% |
67% |
23% |
Figure 1: Clinical Response over Time in Adult Subjects with Severe AA in Trials AA- 1 and AA-2
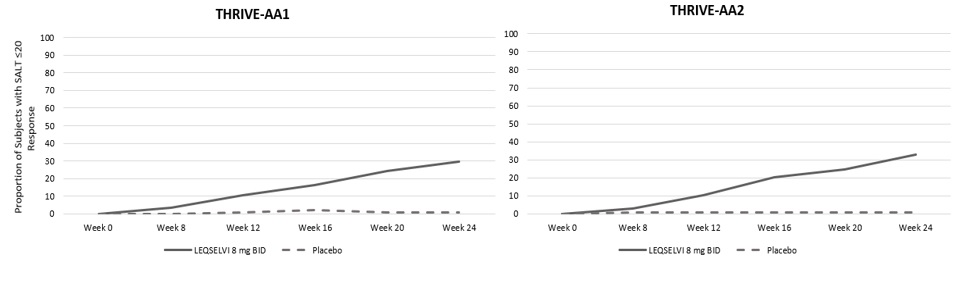
The efficacy of LEQSELVI was generally comparable across multiple subgroups including age, gender, and body weight among these subgroups. The results for SALT score ≤ 20 at Week 24 by baseline scalp hair loss severity are presented in Table 5.
|
Trials AA-1 and AA-2 |
||
|
Placebo |
LEQSELVI |
|
|
50% to 94% Scalp Hair Loss |
||
|
N |
110 |
248 |
|
95% to 100% Scalp Hair Loss |
||
|
N |
157 |
352 |
|
BID = twice-daily |
||
16. How is Leqselvi supplied
How Supplied:
LEQSELVI tablets are packaged in white, high-density polyethylene (HDPE) bottles and closed with 24 mm white child-resistant caps with foil liner. Each bottle contains 1 g silica-gel canister. LEQSELVI is available as purple, round, immediate-release tablets debossed with “C” on one side and “8” on the other side:
- 8 mg: 60 tablets in a bottle; NDC: 47335-108-86
Storage and Handling:
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
Store in the original bottle to protect from moisture.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide)
Serious Infections
Advise patients that they are more likely to develop infections when taking LEQSELVI.
Advise patients that the risk of herpes zoster is increased in patients treated with LEQSELVI and some cases can be serious [see Warnings and Precautions (5.1)].
Instruct patients to tell their healthcare provider if they develop any signs or symptoms of an infection [see Warnings and Precautions (5.1)].
Malignancies and Lymphoproliferative Disorders
Inform patients that LEQSELVI may increase the risk of developing certain cancers, including skin cancers, and that periodic skin examinations should be performed while using LEQSELVI. Instruct patients to inform their healthcare provider if they have ever had any type of cancer [see Warnings and Precautions (5.3)].
Major Adverse Cardiovascular Events
Inform patients that LEQSELVI may increase the risk of major adverse cardiovascular events (MACE) including myocardial infarction, stroke, and cardiovascular death. Instruct all patients, especially current or past smokers or patients with other cardiovascular risk factors, to be alert for the development of signs and symptoms of cardiovascular events [see Warnings and Precautions (5.4)].
Thrombosis
Advise patients that events of DVT, PE and CVT have been reported in clinical trials with LEQSELVI. Instruct patients to seek immediate medical attention if they develop any signs or symptoms of a DVT, PE, or CVT [see Warnings and Precautions (5.5)].
Increased Risk of LEQSELVI-Associated Serious Adverse Reactions in CYP2C9 Poor Metabolizers or with Concomitant Use of Moderate or Strong CYP2C9 Inhibitors
Advise patients to inform their healthcare providers of all medications they are taking, including prescription medicines, over-the-counter drugs, vitamins, and herbal products (e.g., St. John's wort) [see Warnings and Precautions (5.6)].
Gastrointestinal Perforations
Inform patients that gastrointestinal perforations have been reported in clinical trials with LEQSELVI. Instruct patients to seek medical care immediately if they experience new onset of abdominal pain, fever, chills, nausea, or vomiting [see Warnings and Precautions (5.7)].
Laboratory Abnormalities
Inform patients that LEQSELVI may affect certain lab tests, and that blood tests are required before and during LEQSELVI treatment [see Warnings and Precautions (5.8)].
Immunizations
Advise patients that vaccination with live vaccines is not recommended during or immediately prior to LEQSELVI treatment. Instruct patients to inform their healthcare practitioner that they are taking LEQSELVI prior to a potential vaccination [see Warnings and Precautions (5.9)].
Pregnancy
Advise pregnant patients and patients of reproductive potential of the potential risk to a fetus and to inform their healthcare provider if they are pregnant or plan to become pregnant during treatment with LEQSELVI. Inform patients to report their pregnancy to Sun Pharmaceutical Industries, Inc at 1-800-818-4555 [see Use in Specific Populations (8.1) and (8.3)].
Lactation
Advise patients not to breastfeed during treatment with LEQSELVI and for one day after the last dose [see Use in Specific Populations (8.2)].
Manufactured for Sun Pharmaceutical Industries, Inc by: Halo Pharmaceutical, Inc, Whippany, NJ, 07981, USA
U.S. Patent No. 10,561,659; 10,265,258; 11,298,570 and 11,919,907
LEQSELVI is a trademark of Sun Pharmaceutical Industries, Inc.
© 2024 Sun Pharmaceutical Industries, Inc. All rights reserved
Code# 2142-00
7/2024
|
MEDICATION GUIDE
LEQSELVI™ [lek-sel-vee]
|
|
|
What is the most important information I should know about LEQSELVI?
1. Serious Infections. LEQSELVI is a medicine that affects your immune system. LEQSELVI can lower the ability of your immune system to fight infections. Some people have had serious infections during treatment with LEQSELVI, caused by bacteria, fungi or viruses that can spread throughout the body. Some people may die from these infections.
You should not start taking LEQSELVI if you have any kind of infection unless your healthcare provider tells you it is okay. You may be at a higher risk of developing shingles (herpes zoster). Before starting LEQSELVI, tell your healthcare provider if you:
After starting LEQSELVI, call your healthcare provider right away if you have any symptoms of an infection. LEQSELVI can make you more likely to get infections or make any infections that you have worse. If you get a serious infection, your healthcare provider may stop your treatment with LEQSELVI until your infection is controlled. 2. Increased risk of death in people 50 years of age and older who have at least 1 heart disease (cardiovascular) risk factor and are taking a medicine in the class of medicines called Janus kinase (JAK) inhibitors. LEQSELVI is a JAK inhibitor medicine. 3. Cancer and immune system problems. LEQSELVI may increase your risk of certain cancers by changing the way your immune system works.
Tell your healthcare provider if you have ever had any type of cancer. 4. Increased risk of major cardiovascular events such as heart attack, stroke or death in people 50 years of age and older who have at least 1 heart disease (cardiovascular) risk factor and taking a medicine in the class of medicines called JAK inhibitors, especially if you are a current or past smoker. Get emergency help right away if you get any symptoms of a heart attack or stroke during treatment with LEQSELVI, including:
5. Blood clots. Blood clots in the veins of your legs (deep vein thrombosis, DVT), lungs (pulmonary embolism, PE) or brain (cerebral venous sinus thrombosis, CVT) can happen in some people during treatment with LEQSELVI. This may be life-threatening and may cause death. Blood clots in the veins of the legs (DVT) and lungs (PE) have happened more often in people who are 50 years of age and older and with at least 1 heart disease (cardiovascular) risk factor taking a medicine in the class of medicines called Janus kinase (JAK) inhibitors.
6. Tears (perforation) in the stomach or intestines.
7. Changes in certain laboratory test results. Your healthcare provider should do blood tests before you start taking LEQSELVI and during treatment to check for the following:
You should not take LEQSELVI if your white blood cell or red blood cell counts are too low. Your healthcare provider may stop your LEQSELVI treatment for period of time if needed because of changes in these blood test results. See “What are the possible side effects of LEQSELVI?” for more information about side effects. |
|
|
What is LEQSELVI?
|
|
|
Do not take LEQSELVI if you:
|
|
|
Before taking LEQSELVI, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. LEQSELVI and other medicines may affect each other causing side effects. Especially tell your healthcare provider if you take medicines that affect your immune system such as biologic medicines, other JAK inhibitors, and strong immunosuppressants (such as cyclosporine) as these medicines may increase your risk of infection. Ask your healthcare provider or pharmacist if you are not sure if you are taking one of these medicines. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist whenever you get a new medicine. |
|
|
How should I take LEQSELVI?
|
|
|
What are the possible side effects of LEQSELVI?
The most common side effects of LEQSELVI include:
These are not all of the possible side effects of LEQSELVI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
|
How should I store LEQSELVI?
Keep LEQSELVI and all medicines out of the reach of children. |
|
|
General information about the safe and effective use of LEQSELVI. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use LEQSELVI for a condition for which it was not prescribed. Do not give LEQSELVI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about LEQSELVI that is written for health professionals. |
|
|
What are the ingredients in LEQELVI? Active ingredient: deuruxolitinib Inactive ingredients: colloidal silicon dioxide, lactose monohydrate, low-substituted hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose and povidone. The tablet film coat contains: carmine, FD&C blue #2 aluminum lake, glyceryl mono and dicaprylocaprate, polyvinyl alcohol, sodium lauryl sulfate, talc, and titanium dioxide. |
|
|
Manufactured for Sun Pharmaceutical Industries, Inc. by Halo Pharmaceutical Inc., Whippany, NJ, 07981, USA. |
|
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. |
Issued: 7/2024 |
| LEQSELVI
deuruxolitinib phosphate tablet, film coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Sun Pharmaceutical Industries, Inc. (146974886) |
More about Leqselvi (deuruxolitinib)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Imprints, shape & color data
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: multikinase inhibitors
- Breastfeeding
- En español

