Kolorz Sixty Second Fluoride Gel: Package Insert / Prescribing Info
Package insert / product label
Generic name: sodium fluoride
Dosage form: gel
Drug class: Mouth and throat products
Medically reviewed by Drugs.com. Last updated on Sep 26, 2024.
On This Page
Indications and Usage for Kolorz Sixty Second Fluoride Gel
For topical application of fluoride to aid in the protection against dental caries.
Kolorz Sixty Second Fluoride Gel Dosage and Administration
Fill fluoride tray(s) 1/3 full with gel. Dry tooth surface and insert tray(s) in mouth. Have patient bite down for 60 seconds (or up to 4 minutes). Slight chewing motion provides interproximal coverage. Remove tray(s) and have patient expectorate excess gel. Advise patient not to eat, drink or rinse for 30 minutes.
Kolorz Sixty Second Fluoride Gel Description
Sodium Fluoride in a proprietary acidulated phosphate flavored gel base. 1.23% Fluoride Ion from 2.72% Sodium Fluoride.
Sweetened with Sucralose. Does not contain Aspartame or Saccharin. Gluten Free.
Warnings
Keep out of reach of children. Do not swallow. If dosage or less than 100mg is ingested, contact poison control.
Made in USA
Distributed by :DMG America LLC, 242 South Dean Street
Englewood, NJ 07631, 800-662-6383
www.dmg-america.com
Rev. 4/09
RE-ORDER #777304
392415
Related/similar drugs
PRINCIPAL DISPLAY PANEL - 480 ml Bottle Label - Cherry Cheesecake
Best Taste...
Guaranteed!
kolorz®
Sixty
Second
Fluoride Gel
Cherry Cheesecake
APF Topical Fluoride
Acidulated Phosphate Fluoride
1.23% Fluoride Ion
GLUTEN FREE!
Contains
XYLITOL
CAUTION:
For professional use only.
Shake well before each use.
NET WT. 16 OZ (480ml)

PRINCIPAL DISPLAY PANEL - 480 ml Bottle Label - Piña Colada
Best Taste...
Guaranteed!
kolorz®
Sixty
Second
Fluoride Gel
Piña Colada
APF Topical Fluoride
Acidulated Phosphate Fluoride
1.23% Fluoride Ion
GLUTEN FREE!
Contains
XYLITOL
CAUTION:
For professional use only.
Shake well before each use.
NET WT. 16 OZ (480ml)

PRINCIPAL DISPLAY PANEL - 480 ml Bottle Label - Triple Mint
Best Taste...
Guaranteed!
kolorz®
Sixty
Second
Fluoride Gel
Triple Mint
APF Topical Fluoride
Acidulated Phosphate Fluoride
1.23% Fluoride Ion
GLUTEN FREE!
Contains
XYLITOL
CAUTION:
For professional use only.
Shake well before each use.
NET WT. 16 OZ (480ml)

PRINCIPAL DISPLAY PANEL - 480 ml Bottle Label - Blue Raspberry
Best Taste...
Guaranteed!
kolorz®
Sixty
Second
Fluoride Gel
Blue Raspberry
APF Topical Fluoride
Acidulated Phosphate Fluoride
1.23% Fluoride Ion
GLUTEN FREE!
Contains
XYLITOL
CAUTION:
For professional use only.
Shake well before each use.
NET WT. 16 OZ (480ml)

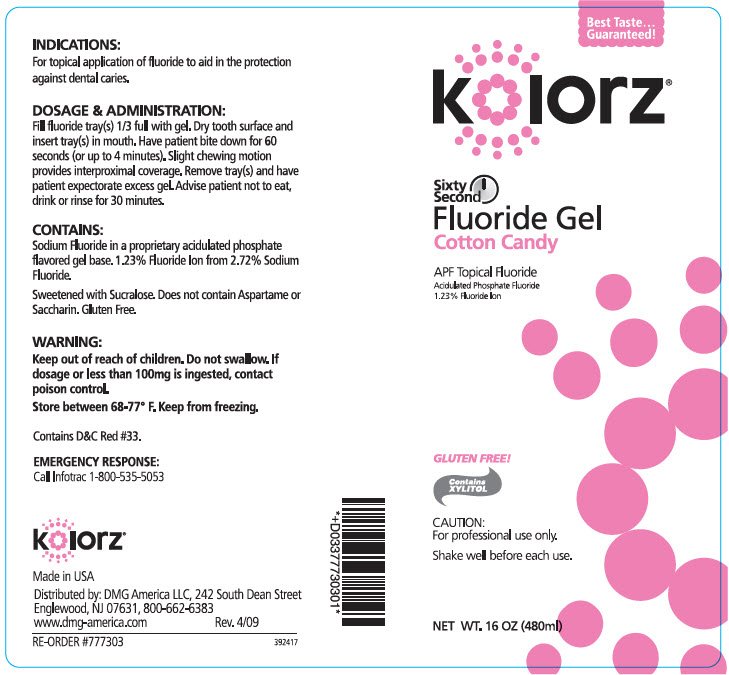
PRINCIPAL DISPLAY PANEL - 480 ml Bottle Label - Cotton Candy
Best Taste...
Guaranteed!
kolorz®
Sixty
Second
Fluoride Gel
Cotton Candy
APF Topical Fluoride
Acidulated Phosphate Fluoride
1.23% Fluoride Ion
GLUTEN FREE!
Contains
XYLITOL
CAUTION:
For professional use only.
Shake well before each use.
NET WT. 16 OZ (480ml)
| KOLORZ SIXTY SECOND FLUORIDE
CHERRY CHEESECAKE
sodium fluoride gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| KOLORZ SIXTY SECOND FLUORIDE
PINA COLADA
sodium fluoride gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| KOLORZ SIXTY SECOND FLUORIDE
TRIPLE MINT
sodium fluoride gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| KOLORZ SIXTY SECOND FLUORIDE
BLUE RASPBERRY
sodium fluoride gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| KOLORZ SIXTY SECOND FLUORIDE
COTTON CANDY
sodium fluoride gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - DMG AMERICA, LLC (106792427) |
More about fluoride topical
- Compare alternatives
- Pricing & coupons
- Reviews (35)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: mouth and throat products
- En español
Patient resources
Professional resources
- 60 Second Fluoride Gel prescribing information
- Fluoride Foam (FDA)
- Sodium Fluoride Dental Cream (FDA)
- Sodium Fluoride Dental Gel (FDA)
- Sodium Fluoride Paste (FDA)
Other brands
Prevident 5000 Plus, Fluoridex, Dentagel, PreviDent 5000 Booster, ... +38 more