Isuprel: Package Insert / Prescribing Info
Package insert / product label
Generic name: isoproterenol hydrochloride
Dosage form: injection, solution
Drug classes: Adrenergic bronchodilators, Catecholamines
Medically reviewed by Drugs.com. Last updated on Mar 3, 2025.
On This Page
Highlights of Prescribing Information
ISUPREL® (isoproterenol hydrochloride) injection, for intravenous use
Initial U.S. Approval: 1956
Indications and Usage for Isuprel
Isuprel Dosage and Administration
Dosage Forms and Strengths
Injection: 0.2 mg/mL and 1 mg/5 mL (0.2 mg/mL) single dose vial (3)
Contraindications
Warnings and Precautions
Adverse Reactions/Side Effects
Common adverse reactions with isoproterenol include tachycardia and palpitations (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- •
- Do not administer ISUPREL and epinephrine simultaneously due to combined effects may induce serious arrhythmias (7)
- •
- Concomitant use of tricyclic antidepressants, monoamine oxidase inhibitors, levothyroxine sodium and certain antihistamines; hemodynamic parameters may potentiate a clinical response of isoproterenol (7)
- •
- Beta-adrenergic blocking drugs may reduce cardiostimulating and bronchodilating effects of isoproterenol (7)
Revised: 1/2023
Full Prescribing Information
1. Indications and Usage for Isuprel
ISUPREL is indicated:
- •
- To improve hemodynamic status in patients in distributive shock and shock due to reduced cardiac output
- •
- For bronchospasm occurring during anesthesia
2. Isuprel Dosage and Administration
2.1 General Considerations
Inspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if the injection is pinkish or darker than slightly yellow or contains a precipitate. Discard any unused portion.
Diluted solution should be used immediately. Unused material should be discarded.
2.2 Recommended Dosage
- Dosage should generally be started at the lowest recommended dose and increased gradually based on patient response.
|
||
|
Recommended dosage for adults with shock and hypoperfusion states: |
||
|
Route of Administration |
Preparation of Dilution* |
Infusion Rate† |
|
Intravenous infusion |
Dilute 5 mL (1 mg) in 500 mL of 5% Dextrose Injection, USP |
0.5 mcg to 5 mcg per minute (0.25 mL to 2.5 mL of diluted solution) |
|
Recommended dosage for adults with bronchospasm occurring during anesthesia: |
|||
|
Route of |
Subsequent |
||
|
Administration |
Preparation of Dilution |
Initial Dose |
Dose |
|
Bolus
|
Dilute 1 mL (0.2 mg) to 10 mL with Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP |
10 mcg to 20 mcg (0.5 mL to 1 mL of diluted solution) |
The initial dose may be repeated when necessary |
There are no well-controlled studies in children to establish appropriate dosing; however, the American Heart Association recommends an initial infusion rate of 0.1 mcg/kg/min, with the usual range being 0.1 mcg/kg/min to 1 mcg/kg/min.
3. Dosage Forms and Strengths
Injection solution: single dose, clear glass ampules containing isoproterenol in a clear, colorless solution;
- •
- 1 mL containing 0.2 mg/1 mL (0.2 mg/mL)
- •
- 5 mL containing 1 mg/5 mL (0.2 mg/mL)
4. Contraindications
ISUPREL is contraindicated in patients with:
- •
- tachycardia
- •
- ventricular arrhythmias
- •
- angina pectoris
5. Warnings and Precautions
5.1 Cardiac Arrhythmias and Ischemia
Isoproterenol may induce cardiac arrhythmias and myocardial ischemia in patients, especially patients with coronary artery disease, or cardiomyopathy.
5.2 Allergic Reactions associated with Sulfite
ISUPREL contains sodium metabisulfite, which may cause mild to severe allergic reactions including anaphylaxis or asthmatic episodes, particularly in patients with a history of allergies. However, the presence of metabisulfite in this product should not preclude its use for treatment in emergency situations, even if the patient is sulfite-sensitive, as the alternatives to using isoproterenol in a life-threatening situation may not be satisfactory.
6. Adverse Reactions/Side Effects
The following adverse reactions have been associated with use of isoproterenol. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Nervous system disorders: Nervousness, headache, dizziness, visual blurring
- Cardiovascular: Tachycardia, tachyarrhythmias, palpitations, angina, ventricular arrhythmias, Adams-Stokes attacks, pulmonary edema
Respiratory: Dyspnea
Other: Flushing of the skin, sweating, mild tremors, pallor, nausea
Related/similar drugs
7. Drug Interactions
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8. Use In Specific Populations
8.1 Pregnancy
- Risk Summary
- Prolonged experience with isoproterenol use in pregnant women over several decades, based on published literature, do not identify a drug associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. However, there are risks to the mother and fetus associated with isoproterenol use during labor or delivery (see Clinical Considerations).
- The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the United States general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
- Clinical Considerations
- Hypotension associated with shock is a medical emergency in pregnancy which can be fatal if left untreated. Delaying treatment in pregnant women with hypotension associated with shock may increase the risk of maternal and fetal morbidity and mortality. Life-sustaining therapy for the pregnant woman should not be withheld due to potential concerns regarding the effects of isoproterenol on the fetus.
- Labor and Delivery
- Isoproterenol usually inhibits spontaneous or oxytocin induced contractions of the pregnant human uterus and may delay the second stage of labor. Avoid isoproterenol during the second stage of labor. Avoid isoproterenol in obstetrics when maternal blood pressure exceeds 130/80 mmHg.
- Although isoproterenol may improve maternal hypotension associated with shock, it may result in uterine vasoconstriction, decreased uterine blood flow, uterine atony with hemorrhage, and fetal anoxia.
8.2 Lactation
Risk Summary
There is no information regarding the presence of isoproterenol in milk or the effects of isoproterenol on the breastfed infant or on milk production. However, due to its short half-life, isoproterenol exposure is expected to be very low in the breastfed infant.
8.4 Pediatric Use
Safety and efficacy of isoproterenol in pediatric patients have not been established.
Intravenous infusions of isoproterenol in refractory asthmatic children at rates of 0.05‑2.7 µg/kg/min have caused clinical deterioration, myocardial necrosis, congestive heart failure and death. The risks of cardiac toxicity appear to be increased by some factors [acidosis, hypoxemia, coadministration of corticosteroids, coadministration of methylxanthines (theophylline, theobromine) or aminophylline] that are especially likely to be present in these patients. If I.V. isoproterenol is used in children with refractory asthma, patient monitoring must include continuous assessment of vital signs, frequent electrocardiography, and daily measurements of cardiac enzymes, including CPK-MB.
8.5 Geriatric Use
Clinical studies of ISUPREL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects in clinical circumstances. There are, however, some data that suggest that elderly healthy or hypertensive patients are less responsive to beta-adrenergic stimulation than are younger subjects. In general, dose selection for elderly patients should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant diseases or other drug therapy.
10. Overdosage
Overdosage of isoproterenol can cause tachycardia or other arrhythmias, palpitations, angina, hypotension, or hypertension. In case of overdosage, reduce the rate of administration or discontinue isoproterenol hydrochloride injection until patient’s condition stabilizes. Monitor blood pressure, pulse, respiration, and EKG.
It is not known whether isoproterenol hydrochloride is dialyzable.
11. Isuprel Description
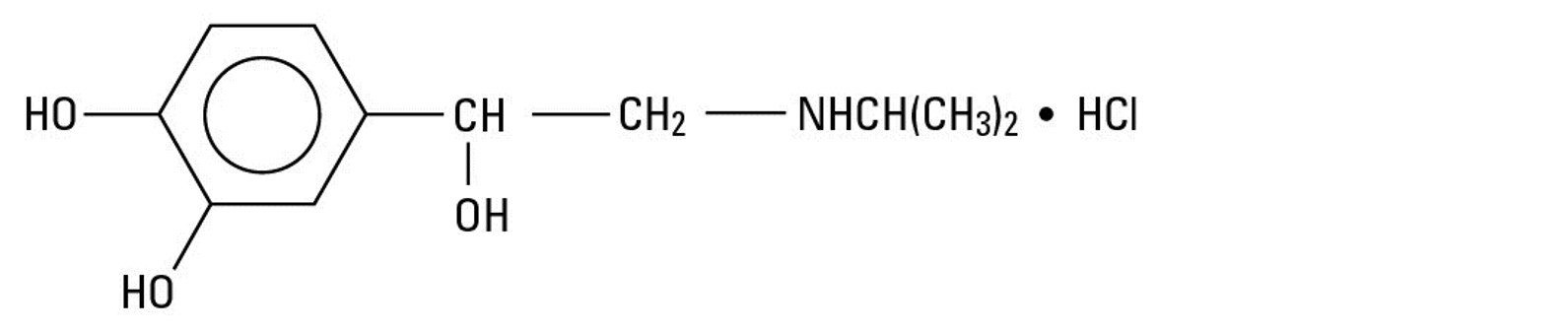
Isoproterenol hydrochloride is 3,4-Dihydroxy-α-[(isopropylamino)methyl] benzyl alcohol hydrochloride, a beta-adrenergic agonist and a synthetic sympathomimetic amine that is structurally related to epinephrine. The molecular formula is C11H17NO3 ● HCl. It has a molecular weight of 247.72 and the following structural formula:●
Isoproterenol hydrochloride is a racemic compound.
|
Each milliliter of the sterile solution contains: |
|
|
Edetate Disodium (EDTA) 0.2 mg |
|
Sodium Citrate Dihydrate 2.07 mg |
|
Citric Acid, Anhydrous 2.5 mg |
|
|
The pH is adjusted between 3.5 and 4.5 with hydrochloric acid and/or sodium hydroxide.
The sterile solution is nonpyrogenic and can be administered by the intravenous route.
12. Isuprel - Clinical Pharmacology
12.1 Mechanism of Action
Isoproterenol is a potent nonselective beta-adrenergic agonist with very low affinity for alpha‑adrenergic receptors.
12.2 Pharmacodynamics
Intravenous infusion of isoproterenol in man lowers peripheral vascular resistance, primarily in skeletal muscle but also in renal and mesenteric vascular beds. Diastolic pressure falls. Renal blood flow is decreased in normotensive subjects but is increased markedly in shock. Systolic blood pressure may remain unchanged or rise, although mean arterial pressure typically falls. Cardiac output is increased because of the positive inotropic and chronotropic effects of the drug in the face of diminished peripheral vascular resistance.
Isoproterenol relaxes almost all varieties of smooth muscle when the tone is high, but this action is most pronounced on bronchial and gastrointestinal smooth muscle. It prevents or relieves bronchoconstriction, but tolerance to this effect develops with overuse of the drug.
In man, isoproterenol causes less hyperglycemia than does epinephrine. Isoproterenol and epinephrine are equally effective in stimulating the release of free fatty acids and energy production.
12.3 Pharmacokinetics
Absorption
Isoproterenol is readily absorbed when given parenterally or as an aerosol.
Elimination
Isoproterenol is metabolized primarily in the liver and other tissues by COMT. Isoproterenol is a relatively poor substrate for MAO and is not taken up by sympathetic neurons to the same extent as are epinephrine and norepinephrine. The duration of action of isoproterenol may therefore be longer than that of epinephrine but is still brief.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate the carcinogenic potential of isoproterenol hydrochloride have not been done. Mutagenic potential and effect on fertility have not been determined. There is no evidence from human experience that isoproterenol hydrochloride injection may be carcinogenic or mutagenic or that it impairs fertility.
16. How is Isuprel supplied
|
NDC Number |
Container |
Concentration |
Fill |
Quantity |
|
0187-4330-01 |
Single-dose ampul |
0.2 mg/mL |
1 mL |
UNI-AMPTM pack of 25 |
|
0187-4330-05 |
Single-dose ampul |
1 mg/5 mL (0.2 mg/mL) |
5 mL |
10 ampuls per carton |
Protect from light. Keep in opaque container until used.
Store at 20º to 25ºC (68º to 77ºF). [See USP Controlled Room Temperature.]
Do not use if the injection is pinkish or darker than slightly yellow or contains a precipitate.
Discard unused portion.
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
ISUPREL is a registered trademark of Hospira, Inc. used under license.
© 2023 Bausch Health Companies Inc. or its affiliates
9669502
| ISUPREL
isoproterenol hydrochloride injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Bausch Health US LLC (831922468) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Avara Liscate Pharmaceutical Services S.p.A | 564165541 | MANUFACTURE(0187-4330) | |
More about Isuprel (isoproterenol)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: adrenergic bronchodilators