Gingimed: Package Insert / Prescribing Info
Package insert / product label
Generic name: stannous fluoride
Dosage form: oral liquid
Drug class: Mouth and throat products
Medically reviewed by Drugs.com. Last updated on Jul 7, 2025.
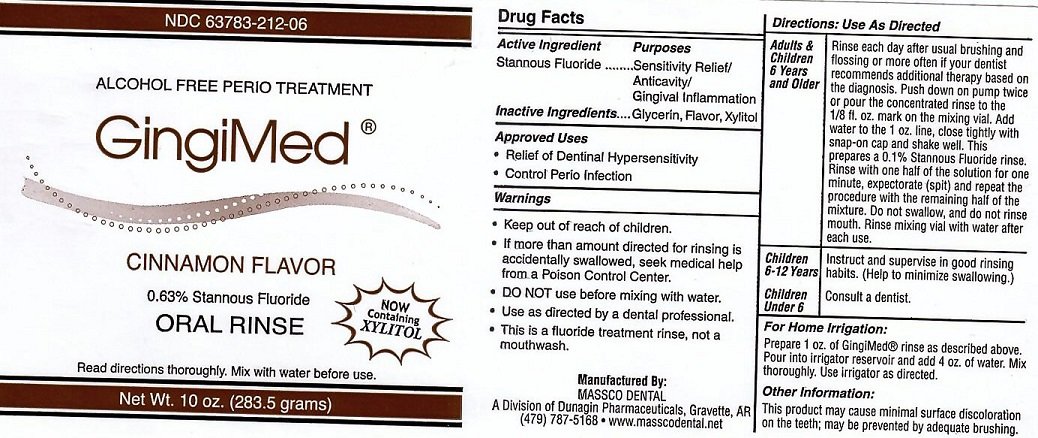
ACTIVE INGREDIENT
STANNOUS FLUORIDE
INACTIVE INGREDIENTS
GLYCERIN, FLAVOR,XYLITOL
Indications and Usage for Gingimed
APPROVED USES: RELIEF OF DENTINAL HYPERSENSITIVITY. CONTROL OF PERIO INFECTION.
KEEP OUT OF REACH OF CHILDREN
KEEP OUT OF REACH OF CHILDREN
Related/similar drugs
DIRECTIONS FOR USE
ADULTS AND CHILDREN 6 YEARS AND OLDER: RINSE EACH DAY AFTER USUAL BRUSHING AND FLOSSING OR MORE OFTEN IF YOUR DENTIST RECOMMENDS ADDITIONAL THERAPY BASED ON THE DIAGNOSIS. PUSH DOWN ON PUMP TWICE OR POUR THE CONCENTRATED RINSE TO THE 1/8 FL. OX. MARK ON THE MIXING VIAL. ADD WATER TO THE 1 OZ. LINE. CLOSE TIGHTLY WITH SNAP-ON CAP AND SHAKE WELL. THIS PREPARES A 0.1% STANNOUS FLUORIDE RINSE. RINSE WITH ONE HALF OF THE SOLUTION FOR ONE MINUTE, EXPECTORATE (SPIT) AND REPEAT THE PROCEDURE WITH THE REMAINING HALF OF THE MIXTURE. DO NOT SWALLOW AND DO NOT RINSE MOUTH. RINSE MIXING VIAL WITH WATER AFTER EACH USE.
CHILDREN 6-12 YEARS: INSTRUCT AND SUPERVISE IN GOOD RINSING HABITS. (HELP TO MINIMIZE SWALLOWING)
CHILDREN UNDER 6: CONSULT A DENTIST.
FOR HOME IRRIGATION: PREPARE 1 OZ. OF GINGIMED RINSE AS DESCRIBED ABOVE. POUR INTO IRRIGATOR RESIVOIR AND ADD 4 OZ. OF WATER. MIX THOROUGHLY. USE IRRIGATOR AS DESCRIBED
OTHER INFORMATION
THIS PRODUCT MAY CAUSE MINIMAL SURFACE DISCOLORATION ON THE TEETH, MAY BE PREVENTED BY ADEQUATE BRUSHING.
Warnings
IF MORE THAN AMOUNT DIRECTED FOR RINSING IS ACCIDENTALLY SWALLOWED, SEEK MEDICAL HELP FROM A POISON CONTROL CENTER. DO NOT USE BEFORE MIXING WITH WATER. USE AS DIRECTED BY A DENTAL PROFESSIONAL. THIS IS A FLUORIDE TREATMENT RINSE, NOT A MOUTHWASH.
PACKAGE LABEL
ALCOHOL FREE PERIO TREATMENT. GINGIMED 0.63% STANNOUS FLUORIDE READ DIRECTIONS THOROUGHLY. MIX WITH WATER BEFORE USE. NOW CONTAINING XYLITOL
MANUFACTURED BY MASSCO DENTAL A DIVISION OF DUNAGIN PHARMACEUTICALS, GRAVETTE, AR (479) 787-5168 WWW.MASSCODENTAL.NET
res
GINGIMED
stannous fluoride liquid |
|
|
|
|
|
|
|
|
|
|
|
|
|
GINGIMED
stannous fluoride liquid |
|
|
|
|
|
|
|
|
|
|
|
|
|
GINGIMED
stannous fluoride liquid |
|
|
|
|
|
|
|
|
|
|
|
|
|
Medical Disclaimer