Clindamycin Pledget: Package Insert / Prescribing Info
Package insert / product label
Generic name: clindamycin phosphate
Dosage form: topical solution
Drug classes: Topical acne agents, Vaginal anti-infectives
Medically reviewed by Drugs.com. Last updated on May 14, 2025.
On This Page
Clindamycin Pledget Description
Clindamycin Phosphate Topical Solution contains clindamycin phosphate, USP, at a concentration equivalent to 10 mg clindamycin per milliliter. Each Clindamycin Phosphate Topical Solution pledget applicator contains approximately 1 mL of topical solution.
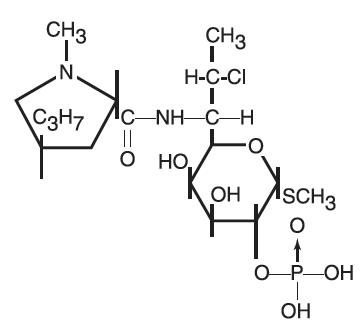
Clindamycin phosphate is a water soluble ester of the semi-synthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent antibiotic lincomycin.
The solution contains isopropyl alcohol 50% v/v, propylene glycol, purified water, and sodium hydroxide (to adjust the pH to between 4.0 - 7.0).
The structural formula is represented below:
The chemical name for clindamycin phosphate is Methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α-D-galacto-octopyranoside 2-(dihydrogen phosphate)
Clindamycin Pledget - Clinical Pharmacology
Although clindamycin phosphate is inactive in vitro, rapid in vivo hydrolysis converts this compound to the antibacterially active clindamycin.
Cross resistance has been demonstrated between clindamycin and lincomycin. Antagonism has been demonstrated between clindamycin and erythromycin.
Following multiple topical applications of clindamycin phosphate at a concentration equivalent to 10 mg clindamycin per mL in an isopropyl alcohol and water solution, very low levels of clindamycin are present in the serum (0-3 ng/mL) and less than 0.2% of the dose is recovered in urine as clindamycin.
Clindamycin activity has been demonstrated in comedones from acne patients. The mean concentration of antibiotic activity in extracted comedones after application of clindamycin phosphate topical solution for 4 weeks was 597 mcg/g of comedonal material (range 0-1490). Clindamycin in vitro inhibits all Propionibacterium acnes cultures tested (MICs 0.4 mcg/mL). Free fatty acids on the skin surface have been decreased from approximately 14% to 2% following application of clindamycin.
Indications and Usage for Clindamycin Pledget
Clindamycin Phosphate Topical Solution USP, 1% is indicated in the treatment of acne vulgaris. In view of the potential for diarrhea, bloody diarrhea and pseudomembranous colitis, the physician should consider whether other agents are more appropriate (see CONTRAINDICATIONS, WARNINGS and ADVERSE REACTIONS).
Contraindications
Clindamycin Phosphate Topical Solution USP, 1% is contraindicated in individuals with a history of hypersensitivity to preparations containing clindamycin or lincomycin, a history of regional enteritis or ulcerative colitis, or a history of antibiotic-associated colitis.
Warnings
Orally and parenterally administered clindamycin has been associated with severe colitis which may result in patient death. Use of the topical formulation of clindamycin results in absorption of the antibiotic from the skin surface. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of topical and systemic clindamycin.
Studies indicate a toxin(s) produced by clostridia is one primary cause of antibiotic-associated colitis. The colitis is usually characterized by severe persistent diarrhea and severe abdominal cramps and may be associated with the passage of blood and mucus. Endoscopic examination may reveal pseudomembranous colitis. Stool culture forClostridium difficile and stool assay for C. difficile toxin may be helpful diagnostically.
When significant diarrhea occurs, the drug should be discontinued. Large bowel endoscopy should be considered to establish a definitive diagnosis in cases of severe diarrhea.
Antiperistaltic agents such as opiates and diphenoxylate with atropine may prolong and/or worsen the condition. Vancomycin has been found to be effective in the treatment of antibiotic-associated pseudomembranous colitis produced by Clostridium difficile. The usual adult dosage is 500 milligrams to 2 grams of vancomycin orally per day in three to four divided doses administered for 7 to 10 days. Cholestyramine or colestipol resins bindvancomycin in vitro. If both a resin and vancomycin are to be administered concurrently, it may be advisable to separate the time of administration of each drug.
Diarrhea, colitis, and pseudomembranous colitis have been observed to begin up to several weeks following cessation of oral and parenteral therapy with clindamycin.
Precautions
General -
Clindamycin Phosphate Topical Solution USP, 1% contains an alcohol base which will cause burning and irritation of the eye. In the event of accidental contact with sensitive surfaces (eye, abraded skin, mucous membranes), bathe with copious amounts of cool tap water. The solution has an unpleasant taste and caution should be exercised when applying medication around the mouth. Clindamycin phosphate topical products should be prescribed with caution in atopic individuals.
Drug Interactions -
Clindamycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. Therefore it should be used with caution in patients receiving such agents.
Pregnancy:
Teratogenic Effects: Pregnancy Category B -
In clinical trials with pregnant women, the systemic administration of clindamycin during the second and third trimesters has not been associated with an increased frequency of congenital abnormalities. There are no adequate studies in pregnant women during the first trimester of pregnancy. Clindamycin should be used during the first trimester of pregnancy only if clearly needed.
Nursing Mothers -
It is not known whether clindamycin is excreted in human milk following use of Clindamycin Phosphate Topical Solution USP, 1%. However, orally and parenterally administered clindamycin has been reported to appear in breast milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use -
Safety and effectiveness in pediatric patients under the age of 12 have not been established.
Geriatric Use -
Clinical studies for clindamycin phosphate topical solution USP, 1% did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Adverse Reactions/Side Effects
In 18 clinical studies of various formulations of topical clindamycin phosphate using placebo vehicle and/or active comparator drugs as controls, patients experienced a number of treatment emergent adverse dermatologic events [see table below].
|
Number of Patients Reporting Events |
|||
|
Treatment Emergent |
Solution |
Gel |
Lotion |
|
Adverse Event |
n=553(%) |
n=148(%) |
n=160(%) |
|
Burning |
62 (11) |
15 (10) |
17 (11) |
|
Itching |
36 (7) |
15 (10) |
17 (11) |
|
Burning/Itching |
60 (11) |
# (-) |
# (-) |
|
Dryness |
105 (19) |
34 (23) |
29 (18) |
|
Erythema |
86 (16) |
10 (7) |
22 (14) |
|
Oiliness/Oily Skin |
8 (1) |
26 (18) |
12* (10) |
|
Peeling |
61 (11) |
# (-) |
11 (7) |
|
# not recorded | |||
|
* of 126 subjects | |||
Orally and parenterally administered clindamycin has been associated with severe colitis which may end fatally. Cases of diarrhea, bloody diarrhea and colitis (including pseudomembranous colitis) have been reported as adverse reactions in patients treated with oral and parenteral formulations of clindamycin and rarely with topical clindamycin (see WARNINGS).
Abdominal pain, gastrointestinal disturbances, gram-negative folliculitis, eye pain and contact dermatitis have also been reported in association with the use of topical formulations of clindamycin.
To report SUSPECTED ADVERSE REACTIONS, contact Padagis at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Related/similar drugs
Overdosage
Topically applied Clindamycin Phosphate Topical Solution USP, 1% can be absorbed in sufficient amounts to produce systemic effects (see WARNINGS).
Clindamycin Pledget Dosage and Administration
Do not use if the seal on jar is broken. Remove pledget from jar just before use. Use a pledget to apply a thin film of Clindamycin Phosphate Topical Solution to the affected area twice daily. More than one pledget may be used. Each pledget should be used only once and then discarded. Keep jar tightly closed when not in use.
How is Clindamycin Pledget supplied
Clindamycin Phosphate Topical Solution USP, 1% is available as follows:
A jar containing 60 single-use pledget applicators (NDC 45802-263-37)
A jar containing 69 single-use pledget applicators (NDC 45802-263-93)
Storage and Handling
Store at 20-25°C (68-77°F) [see USP Controlled Room Temperature]. Protect from freezing.
Manufactured By Padagis
Yeruham, Israel
Distributed By Padagis
Allegan, MI 49010
www.padagis.com
Rev 11-22
74P33 RC F3
Package/Label Display Panel
Rx Only
NDC 45802-263-37
Clindamycin Phosphate Topical Solution USP, 1%* (Pledgets)
*equivalent to 1% (10 mg/mL) clindamycin
For Topical Use Only
60 Pledgets
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
| CLINDAMYCIN PHOSPHATE
clindamycin phosphate solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Padagis Israel Pharmaceuticals Ltd (600093611) |
More about clindamycin topical
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (232)
- Side effects
- Dosage information
- During pregnancy
- Drug class: topical acne agents
- Breastfeeding
Patient resources
Professional resources
- Clindamycin Phosphate (Topical) monograph
- Clindacin Foam (FDA)
- Clindamycin Foam (FDA)
- Clindamycin Gel (FDA)
- Clindamycin Lotion (FDA)
Other brands
Clindagel, Cleocin Vaginal, Clindamax, Xaciato, ... +4 more