Accrufer: Package Insert / Prescribing Info
Package insert / product label
Generic name: ferric maltol
Dosage form: capsule
Drug class: Iron products
Medically reviewed by Drugs.com. Last updated on Dec 13, 2023.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
ACCRUFER (ferric maltol) capsules, for oral use
Initial U.S. Approval: 2019
Indications and Usage for Accrufer
ACCRUFER is an iron replacement product indicated for the treatment of iron deficiency in adults. ( 1)
Accrufer Dosage and Administration
Dosage Forms and Strengths
Capsules: 30 mg ( 3)
Contraindications
Warnings and Precautions
Adverse Reactions/Side Effects
Most common adverse reactions (incidence > 1%) are flatulence, diarrhea, constipation, feces discolored, abdominal pain, nausea, vomiting and abdominal discomfort/distension. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Shield Therapeutics Inc at 1-888-963-6267 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2023
Full Prescribing Information
1. Indications and Usage for Accrufer
ACCRUFER is indicated for the treatment of iron deficiency in adults.
2. Accrufer Dosage and Administration
2.1 Recommended Dosage
The recommended dosage of ACCRUFER is 30 mg twice daily, taken 1 hour before or 2 hours after a meal. Do not open, break, or chew ACCRUFER capsules.
Treatment duration will depend on the severity of iron deficiency but generally at least 12 weeks of treatment is required. The treatment should be continued as long as necessary until ferritin levels are within the normal range.
3. Dosage Forms and Strengths
Capsules: ACCRUFER contains 30 mg iron, as ferric maltol, in red capsules printed with “30”.
4. Contraindications
ACCRUFER is contraindicated in patients with a history of:
- Hypersensitivity to the active substance or to any of the excipients [see Description ( 11)] . Reactions could include shock, clinically significant hypotension, loss of consciousness, and/or collapse.
- Hemochromatosis and other iron overload syndromes [see Warnings and Precautions ( 5.1)] . Use may result in iron overdose [see Overdosage ( 10)].
- Receiving repeated blood transfusions. Use may result in iron overload [see Warnings and Precautions ( 5.2) and Overdosage ( 10)].
5. Warnings and Precautions
5.1 Increased Risk of Inflammatory Bowel Disease (IBD) Flare
Avoid use of ACCRUFER in patients with an active inflammatory bowel disease (IBD) flare, as there is potential risk of increased inflammation in the gastrointestinal tract.
5.2 Iron Overload
Excessive therapy with iron products can lead to excess storage of iron with the possibility of iatrogenic hemosiderosis. Do not administer ACCRUFER to patients with evidence of iron overload or patients receiving intravenous iron [see Contraindications( 4)] . Assess iron parameters prior to initiating ACCRUFER and monitor iron parameters while on therapy [see Overdosage ( 10) and Clinical Pharmacology ( 12.2)] .
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Increased Risk of Inflammatory Bowel Disease Flare [see Warnings and Precautions ( 5.1)]
- Iron Overload [see Warnings and Precautions ( 5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to ACCRUFER in 175 patients in the placebo-controlled phase of three randomized studies conducted in patients with anemia and quiescent inflammatory bowel disease (IBD) (Studies AEGIS 1 & 2) or non-dialysis dependent chronic kidney disease (CKD) (AEGIS 3). The pooled patient population had a mean age of 58 years, 67.4% were female (n=118), and 81.7% (n=143) were Caucasian.
Table 1 presents all adverse reactions occurring in the placebo-controlled period of the pooled randomized studies [see Clinical Studies ( 14)] occurring at a rate of > 1% in the treated group, and for which the rate for ACCRUFER exceeds the rate for placebo.
| ACCRUFER
30 mg bid (N = 175) | Placebo
(N = 120) |
||
|
Body System | |||
| Adverse Reaction | |||
| Gastrointestinal | |||
| Flatulence | 4.6% | 0.0% | |
| Diarrhea | 4.0% | 1.7% | |
| Constipation | 4.0% | 0.8% | |
| Feces discolored | 4.0% | 0.8% | |
| Abdominal pain | 2.9% | 2.5% | |
| Nausea | 1.7% | 0.8% | |
| Vomiting | 1.7% | 0.0% | |
| Abdominal Discomfort | 1.1% | 0.0% | |
| Abdominal Distension | 1.1% | 0.0% | |
The proportion of patients who discontinued treatment due to adverse reactions during the double-blind, placebo-controlled portion of studies was 4.6% for patients taking ACCRUFER. The most common adverse reaction leading to discontinuation of ACCRUFER in these studies was abdominal pain (1.7% of patients).
Related/similar drugs
7. Drug Interactions
7.1 Effect of Other Drugs on ACCRUFER
Oral Medications
There are no empirical data on avoiding drug interactions between ACCRUFER and concomitant oral medications. Concomitant use of some drugs may reduce the bioavailability of iron after administration of ACCRUFER. Separate the administration of ACCRUFER from these drugs. The duration of separation may depend on the absorption characteristics of the medication concomitantly administered, such as time to peak concentration or whether the drug is an immediate or extended release product. Monitor clinical response to ACCRUFER.
7.2 Effect of ACCRUFER on Other Drugs
Dimercaprol
Concomitant use of iron products with dimercaprol may increase the risk of nephrotoxicity. Avoid concomitant use of ACCRUFER with dimercaprol.
Oral Medications
Concomitant use of ACCRUFER may decrease the bioavailability of some drugs, including mycophenolate, ethinyl estradiol, ciprofloxacin and doxycycline [see Clinical Pharmacology ( 12.3)] . For oral drugs where reductions in bioavailability may cause clinically significant effects on its safety or efficacy, separate the administration of ACCRUFER by at least 4 hours. Monitor clinical responses to concomitant drugs as appropriate.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
ACCRUFER is not absorbed systemically as an intact complex following oral administration, and maternal use is not expected to result in fetal exposure to the drug [see Clinical Pharmacology ( 12.3)] .
In animal reproduction studies, oral administration of ferric or ferrous compounds to gravid CD1-mice and Wistar-rats during organogenesis at doses 13 to 32 times the recommended human dose resulted in no adverse developmental outcomes. An overdose of iron in pregnant women may carry a risk for spontaneous abortion, gestational diabetes and fetal malformation.
In animal reproduction studies, oral administration of maltol to pregnant Crl: COBS-CD (SD) BR rats during organogenesis at doses 6 times the recommended human dose resulted in no adverse developmental outcomes.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Untreated iron deficiency anemia (IDA) in pregnancy is associated with adverse maternal outcomes such as post-partum anemia. Adverse pregnancy outcomes associated with IDA include increased risk for preterm delivery and low birth weight.
Data
Animal Data
In embryofetal development studies in mice and rats, pregnant animals received oral doses of ferric or ferrous compounds (ferrous sulfate or ferric sodium pyrophosphate) of up to 160 mg/kg/day in mice, or up to 200 mg/kg/day in rats, during the period of organogenesis. Administration of ferric or ferrous compounds at doses 13 times (in mice) or 32 times (in rats) the recommended human dose resulted in no maternal toxicity and no adverse developmental outcomes.
In a multigeneration reproductive and developmental study in rats, pregnant animals received oral doses of maltol of 100, 200, and 400 mg/kg/day, during the period of organogenesis. Administration of maltol at doses 6 times the recommended human dose resulted in no maternal toxicity and no adverse developmental outcomes.
8.2 Lactation
Risk Summary
There are no data on the presence of ACCRUFER in human milk, the effects on the breastfed child, or the effects on milk production. ACCRUFER is not absorbed systemically as an intact complex by the mother following oral administration, and breastfeeding is not expected to result in exposure of the child to ACCRUFER.
10. Overdosage
No data are available regarding overdose of ACCRUFER in patients. Acute iron ingestion of 20 mg/kg elemental iron is potentially toxic and 200- 250 mg/kg is potentially fatal. Early signs and symptoms of iron overdose may include nausea, vomiting, abdominal pain and diarrhea. In more serious cases there may be evidence of hypoperfusion, metabolic acidosis and systemic toxicity.
Dosages of ACCRUFER in excess of iron needs may lead to accumulation of iron in storage sites leading to hemosiderosis. Periodic monitoring of iron parameters such as serum ferritin and transferrin saturation may assist in recognizing iron accumulation. Do not administer ACCRUFER to patients with iron overload [see Contraindications ( 4) ].
11. Accrufer Description
ACCRUFER (ferric maltol) capsules, an iron replacement product for oral administration, contain 30 mg iron and 201.5 mg maltol. Ferric maltol contains iron in a stable ferric state as a complex with a trimaltol ligand. Ferric maltol is 3-hydroxy-2-methyl-4H-pyrane-4-one iron (III) complex (3:1) and has the molecular formula (C 6H 5O 3) 3Fe and a molecular mass of 431.2.
Each red capsule, printed with “30”, contains colloidal anhydrous silica, crospovidone (Type A), lactose monohydrate, magnesium stearate and sodium lauryl sulfate as inactive ingredients. In addition, the capsule shell contains FD&C Blue No. 1, FD&C Red No. 40, FD&C Yellow No.6, hypromellose and titanium dioxide. The ink used for printing the marking contains ammonium hydroxide, ethanol, iron oxide black and propylene glycol.
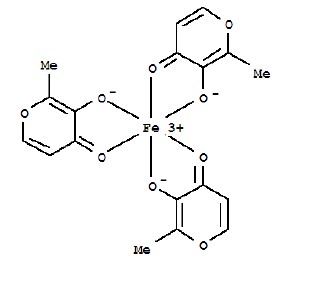
12. Accrufer - Clinical Pharmacology
12.1 Mechanism of Action
ACCRUFER delivers iron for uptake across the intestinal wall and transfer to transferrin and ferritin.
12.2 Pharmacodynamics
ACCRUFER has been shown to increase serum iron parameters, including ferritin and transferrin saturation (TSAT).
12.3 Pharmacokinetics
The pharmacokinetic properties of serum iron after administration of ACCRUFER was assessed in subjects with iron deficiency (with or without anemia) following a single dose and at steady state (after 1 week) of ACCRUFER 30 mg, 60 mg, or 90 mg twice daily (1 to 3 times the approved recommended dosage). Total serum iron concentrations increase in a less than dose proportional manner with increasing ACCRUFER doses.
Absorption
ACCRUFER dissociates upon uptake from the gastrointestinal tract allowing iron and maltol to be absorbed separately.
Total serum iron peak values were reached 1.5 to 3 hours after administration of ACCRUFER, and were comparable between Day 1 and Day 8.
Effect of Food
Food has been shown to decrease the bioavailability of iron after administration of ferric maltol.
Drug Interaction Studies
In vitro
Of the drugs screened for an interaction with ferric maltol in vitro at pH 1.2, 4.5 and 6.8, only mycophenolate and ethinyl estradiol showed any potential for interaction. Mycophenolate recovery was reduced by up to 16% at pH 1.2 but there was no interaction at pH 4.5; due to solubility issues data are not available for pH 6.8. Ethinyl estradiol recovery was reduced by up to 35% at pH 4.5; due to solubility issues data are not available for pH 1.2 and pH 6.8. These potential oral interactions can be avoided by spacing the administration of those drugs and ACCRUFER [ see Drug Interactions ( 7.2)] .
Lisinopril, metoprolol and warfarin showed no interaction at any of the 3 pH conditions and can be taken with ACCRUFER.
No interaction with ferric maltol was observed for atorvastatin (pH 6.8), and norgestimate (pH 1.2) (data were not obtainable at the other pH conditions due to solubility issues).
In vivo
No clinical studies evaluating the drug interaction potential of ACCRUFER have been conducted.
Iron-containing preparations may decrease ciprofloxacin absorption into the bloodstream, resulting in lower serum and urine levels and reduced effectiveness.
Absorption of tetracyclines including doxycycline is reported to be impaired by iron-containing preparations.
12.6 Maltol Phamacokinetics
Maltol is metabolized through glucuronidation (UGT1A6) and sulphation in vitro. Of the total maltol ingested, a mean of between 39.8% and 60% was excreted in the urine as maltol glucuronide. There was no clinically meaningful change in exposure of maltol or maltol glucuronide in subjects with non-dialysis dependent chronic kidney disease (eGFR of >15 mL/min/1.73m 2and <60 mL/min/1.73m 2).
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Ferric maltol
ACCRUFER is not absorbed systemically as an intact complex.
Carcinogenicity studies have not been conducted with ferric maltol.
Ferric maltol was mutagenic in vitro in reverse bacterial mutation (Ames) assays. Ferric maltol increased revertant frequency in the absence and presence of metabolic activation.
Fertility studies have not been conducted with ferric maltol.
Maltol
The carcinogenic potential of maltol has been evaluated in long-term animal toxicity studies in two species: CD-1 mice and Sprague-Dawley rats. Maltol was not carcinogenic in a 18-month study in mice at doses up to 400 mg/kg (approximately 5 times the human daily dose). Maltol was not carcinogenic in a 2-year study in rats at doses up to 400 mg/kg (approximately 10 times the human daily dose).
Maltol was mutagenic in vitro in reverse bacterial mutation (Ames) assays. Maltol increased revertant frequency in the absence and presence of metabolic activation. Maltol was clastogenic in vivo in a mouse micronucleus assay (increase in polychromatic erythrocytes) at intraperitoneal doses of 774 mg/kg. Absorbed maltol is rapidly conjugated with glucuronic acid. It is therefore unlikely that the mutagenic activity of maltol would be expressed under the conditions of oral human intake.
In a multi-generation animal reproduction study in male and female rats, there were no effects on mating, fertility, or early embryonic development at doses up to 400 mg/kg/day (approximately 10 times the human daily dose).
14. Clinical Studies
14.1 Patients with Inflammatory Bowel Disease (IBD)
The safety and efficacy of ACCRUFER for the treatment of iron deficiency anemia was studied in two randomized, placebo-controlled trials: AEGIS 1 (NCT01252221) and AEGIS 2 (NCT01340872). These trials enrolled 128 patients (age range 18-76 years; 45 males and 83 females) with quiescent IBD (58 patients with Ulcerative Colitis [UC] and 70 patients with Crohns disease [CD]) and baseline Hb concentrations between 9.5 g/dL and 12 /13 g/dL for females / males and ferritin < 30 mcg/L. All patients had discontinued prior oral ferrous product treatment due to lack of efficacy or inability to tolerate oral iron replacement products. Subjects were randomized 1:1 to receive either 30 mg ACCRUFER twice daily or a matched placebo control for 12 weeks.
The major efficacy outcome was the mean difference in Hb concentration from baseline to week 12 between ACCRUFER and placebo. The Least Square [LS] mean difference from baseline was 2.18 g/dL (p<0.0001)(see Table 2).
|
Visit (Week) Statistic | ACCRUFER
(N = 64) | Placebo
(N =64) |
|
| Baseline | |||
| Mean (SD) | 11.0 (1.03) | 11.10 (0.85) | |
| Mean change from baseline to Week 12 | |||
| LS Mean (SE) | 2.25 (0.12) | 0.06 (0.13) | |
| Treatment Comparison | Difference in Change From Baseline | ||
| LSM Difference (SE)
ACCRUFER Placebo) | 1-sided lower
97.5%CI | p-value | |
| ACCRUFER versus placebo | 2.18 (0.19) | (1.81) | <0.0001 |
| Note: Multiple imputation was based on treatment, gender, disease [UC or CD], and Hb concentration at baseline, Week 4, and 8. For each imputed dataset, the change from baseline to Week 12 was analyzed using an ANCOVA model with treatment as the factor and gender, disease, baseline Hb concentration as covariates. | |||
The LS mean difference in change from baseline Hb to Week 4 and 8 between ACCRUFER and placebo were 1.04 g/dl and 1.73 g/dl, respectively.
The mean ferritin (mcg/L) levels in ACCRUFER subjects at baseline were 8.6 mcg/L [SD 6.77]) and the mean ferritin (mcg/L) levels at Week 12 were 26.0 mcg/L [SD 30.57] with a mean overall improvement of 17.3 mcg/L.
Following completion of the 12-week placebo-controlled phase of the studies, eligible patients transitioned to ACCRUFER 30 mg twice daily open-label treatment for an additional 52 weeks.
During the open-label phase with ACCRUFER, the mean change in Hb concentration from baseline to Week 64 was 3.1 g/dL [SD 1.46 g/dL, n = 35] and the ferritin value demonstrated a mean of 68.9 mcg/L [SD 96.24] at 64 weeks, with a mean overall improvement of 60.4 mcg/L.
14.2 Patients with Chronic Kidney Disease (CKD)
The safety and efficacy of ACCRUFER for the treatment of iron deficiency anemia was studied in AEGIS 3 (NCT02968368), a trial that enrolled 167 patients (mean age 67.4 years, range 30-90 years; 50 males and 117 females) with non-dialysis dependent chronic kidney disease (CKD) and baseline hemoglobin (Hb) concentrations between 8g/dL and 11 g/dL and ferritin < 250 mcg/L with a Transferrin saturation (TSAT) <25% or ferritin < 500 mcg/L with a TSAT <15%. ACCRUFER was administered at a dose of 30 mg twice daily. Subjects were randomized 2:1 to receive either 30 mg ACCRUFER twice daily or a matched placebo control for 16 weeks.
The major efficacy outcome was the mean difference in Hb concentration from baseline to Week 16 between ACCRUFER and placebo. The LS mean difference was 0.52 g/dL (p= 0.0149) (see Table 3).
|
Visit (Week) Statistic | ACCRUFER
(N = 111) | Placebo
(N = 56) |
|
| Baseline | |||
| Mean (SD) | 10.06 (0.77) | 10.03 (0.82) | |
| Mean change from baseline to Week 16 | |||
| LS Mean (SE) | 0.50 (0.12) | -0.02 (0.16) | |
| Treatment Comparison | Difference in Change From Baseline | ||
| LSM Difference (SE)
ACCRUFER – Placebo | 95% CI | p-value | |
| ACCRUFER versus placebo | 0.52 (0.21) | (0.10, 0.93) | 0.0149 |
| Note: Multiple imputation was based on treatment, gender, eGFR at baseline, and Hb concentration at baseline, Week 4 and 8. For each imputed dataset, the change from baseline to Week 16 was analyzed using an ANCOVA model with treatment as the factor and baseline Hb concentration, baseline eGFR as covariates. | |||
The LS mean difference in change from baseline Hb to Week 4 and 8 between ACCRUFER and placebo were 0.13 g/dl and 0.46 g/dl, respectively.
The mean change in ferritin concentration from baseline to Week 16 was 49.3 mcg/L for the ACCRUFER group and 6.3 mcg/L for the placebo group. The mean difference for ACCRUFER versus placebo was 43.0 mcg/L.
16. How is Accrufer supplied
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Dosing Recommendations
Inform patients to take ACCRUFER as directed on an empty stomach, at least 1 hour before or 2 hours after meals. Instruct patients on concomitant medications that should be dosed apart from ACCRUFER [see Dosage and Administration ( 2.1) and Drug Interactions ( 7.2)] .
Adverse Reactions
Advise patients that ACCRUFER may cause, flatulence, diarrhea, constipation, discolored feces, abdominal pain, nausea, vomiting or abdominal bloating or discomfort. Advise patients to report severe or persistent gastrointestinal symptoms or any allergic reactions to their physician [see Adverse Reactions ( 6.1)].
Increased Risk of IBD Flare
Advise patients that they should not use ACCRUFER if they are experiencing an IBD flare.
Iron Overload and Risk of Accidental Overdose in Children
Inform patients to keep this product out of reach of children as accidental over dose of iron products is a leading cause of fatal poisonings in children. In case of accidental overdose, advise them to call a doctor or poison control center immediately [see Warnings and Precautions ( 5.2)].
Distributed by Shield Therapeutics Inc, Suite 200, 100 Worcester Street, Wellesley Hills, MA, 02481
Patient Information
ACCRUFER
®(ak-roo-fer)
(ferric maltol)
capsules
What is ACCRUFER?
ACCRUFER is a prescription medicine used in adults to treat low iron stores in your body.
It is not known if ACCRUFER is safe and effective for use in children.
Do not takeACCRUFER if you:
- are allergic to ferric maltol or any of the ingredients in ACCRUFER. See the end of this leaflet for a complete list of ingredients in ACCRUFER.
- have any illness that causes you to store too much iron in your body or if you have a problem with how your body uses iron.
- are receiving repeated blood transfusions.
Before taking ACCRUFER, tell your healthcare provider about all your medical conditions, including if you:
- have inflammatory bowel disease (IBD).
- are pregnant or plan to become pregnant. It is not known if ACCRUFER will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if ACCRUFER passes into your breast milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby during treatment with ACCRUFER.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking ACCRUFER with certain other medicines may affect each other causing serious side effects.
Some medicines may need to be taken at least 4 hours before or 4 hours after you have taken your ACCRUFER dose. Ask your healthcare provider for a list of these medicines if you are not sure if you take one of these medicines.
Especially tell your healthcare provider if you take:
- dimercaprol
- other oral iron tablets or health supplements containing iron
Ask your healthcare provider if you are not sure if you take one of these medicines.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I takeACCRUFER?
- Take ACCRUFER exactly as your healthcare provider tells you to.
- Take ACCRUFER 2 times a day on an empty stomach 1 hour before or 2 hours after meals.
- Swallow ACCRUFER capsules whole. Do notopen, break, or chew ACCRUFER capsules.
- In case of accidental overdose, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of ACCRUFER?
ACCRUFER may cause serious side effects, including:
- Increased risk of inflammatory bowel disease (IBD) flare.You should avoid taking ACCRUFER if you have inflammatory bowel disease (IBD) and are experiencing a flare.
- Too much iron stored in your body (iron overload).Your healthcare provider should check the iron level in your blood before you start and during treatment with ACCRUFER.
- Risk of overdose in children due to accidental swallowing.Accidental overdose of iron-containing products is a leading cause of death from poisoning in children under 6. Keep ACCRUFER in a safe place and out of the reach of children.
The most common side effects of ACCRUFER include:
|
|
|
|
|
|
|
These are not all the possible side effects of ACCRUFER.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I storeACCRUFER?
- Store ACCRUFER at room temperature between 68°F to 77°F (20°C to 25°C).
Keep ACCRUFER and all medicines out of reach of children.
General information about the safe and effective use of ACCRUFER.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ACCRUFER for a condition for which it was not prescribed. Do not give ACCRUFER to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about ACCRUFER that is written for health professionals.
What are the ingredients in ACCRUFER?
Active ingredient:ferric maltol
Inactive ingredients:
Capsule:colloidal anhydrous silica, crospovidone (Type A), lactose monohydrate, magnesium stearate, sodium lauryl sulfate
Capsule Shell:FD&C Blue No. 1 FD&C Red No. 40, FD&C Yellow 6, hypromellose, titanium dioxide.
Ink:ammonium hydroxide, ethanol, iron oxide black, propylene glycol
Distributed by Shield Therapeutics Inc, Suite 200, 100 Worcester Street, Wellesley Hills, MA, 02481
US Patents 7459569, 9248148, 9802973, 10179120
This Patient Information has been approved by the U.S. Food and Drug Adminstration. Issued: 10/2023
PRINCIPAL DISPLAY PANEL - 60 Capsules
Carton Label - ACCRUFER ®(ferric maltol) capsules 30 mg (60 Capsules)
NDC 73059-001-60
ACCRUFeR
®
(ferric maltol*)
capsules
30 mg
For oral
administration only
60 capsules
Distributed by
Shield Therapeutics Inc
SHIELD
THERAPEUTICS
Rx only

Bottle Label - ACCRUFER ®(ferric maltol) capsules 30 mg
NDC73059-001-60
ACCRUFeR
®
(ferric maltol*)
capsules
30 mg
For oral administration only
60 capsules
SHIELD
THERAPEUTICS
Rx only
*Each capsule contains 30 mg iron (as
ferric maltol)
WARNING:Accidental overdose of
iron-containing products is a leading
cause of fatal poisoning in children
under 6. Keep this product out of reach
of children. In case of accidental
overdose, call a doctor or poison control
center immediately.
Store at 20°C to 25°C (68°F to 77°F);
excursions permitted to 15°C to 30°C
(59°F to 86°F). (See USP controlled room
temperature).
Usual Dosage: 1 capsule twice daily on an
empty stomach. See prescribing information.
Distributed by Shield Therapeutics Inc.

PRINCIPAL DISPLAY PANEL - 10 Capsules
Carton Label - ACCRUFER ® (ferric maltol) capsules 30 mg (10 Capsules)
NDC 73059-001-10
ACCRUFeR®
(ferric maltol*)
capsules
30 mg
For oral
administration only
10 capsules
Professional Sample -
Not For Sale
SHIELD
THERAPEUTICS
Rx only
Bottle Label - ACCRUFER ® (ferric maltol) capsules 30 mg (10 Capsules)
NDC 73059-001-10
ACCRUFeR®
(ferric maltol*) 30 mg capsules
For oral administration
only
10 capsules
Sample-Not for Sale
SHIELD
THERAPEUTICS
Rx only
*Each capsule contains 30 mg iron (as ferric maltol).
Store at 20°C to 25°C (68°F to 77°F);
Usual Dosage: 1 capsule twice daily on an
empty stomach. See Prescribing Information.
Distributed by Shield Therapeutics Inc.

| ACCRUFER
ferric maltol capsule |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Shield TX (UK) Ltd (211237588) |
| Registrant - Patheon France S.A.S (543127229) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Butterworth Laboratories Ltd | 225081538 | analysis(73059-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Amatsi Analytics | 270324185 | analysis(73059-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sterling Pharma Solutions Limited | 349157623 | api manufacture(73059-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ACM Pharma | 384719527 | analysis(73059-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon France S.A.S | 543127229 | manufacture(73059-001) , analysis(73059-001) , pack(73059-001) , label(73059-001) | |
More about Accrufer (ferric maltol)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Imprints, shape & color data
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: iron products
- En español
