M 315 Pill: white, round, 6mm
The pill with imprint M 315 (White, Round, 6mm) has been identified as Ondansetron Hydrochloride 4 mg and is used for Nausea/Vomiting, Nausea/Vomiting, Postoperative, Nausea/Vomiting, Chemotherapy Induced, and Nausea/Vomiting, Radiation Induced. It belongs to the drug class 5HT3 receptor antagonists and is not a controlled substance.
Images for M 315
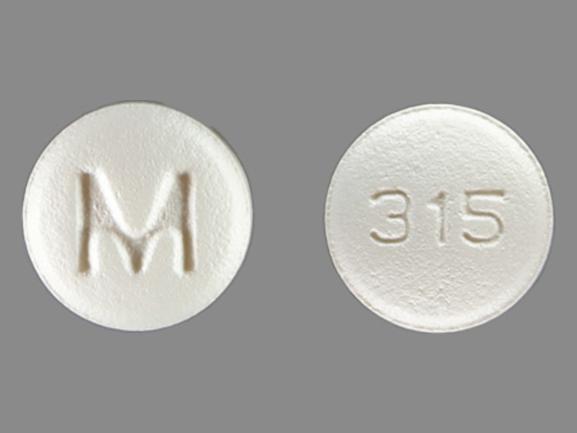
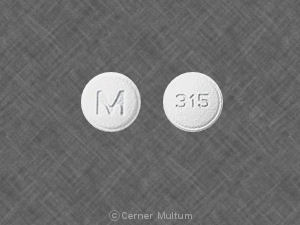

Ondansetron Hydrochloride
- Imprint
- M 315
- Strength
- 4 mg
- Color
- White
- Size
- 6.00 mm
- Shape
- Round
- Availability
- Prescription only
- Drug Class
- 5HT3 receptor antagonists
- Pregnancy Category
- B - No proven risk in humans
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Mylan Pharmaceuticals Inc.
- Inactive Ingredients
-
lactose anhydrous,
silicon dioxide,
croscarmellose sodium,
hypromelloses,
magnesium stearate,
microcrystalline cellulose,
polydextrose,
polyethylene glycol,
corn starch,
sodium lauryl sulfate,
titanium dioxide,
triacetin
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00378-0315 (Discontinued) | Mylan Pharmaceuticals Inc. |
| 51079-0524 (Discontinued) | UDL Laboratories Inc. |
Related images for "M 315"
More about ondansetron
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (503)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: 5HT3 receptor antagonists
- Breastfeeding
Patient resources
- Ondansetron drug information
- Ondansetron injection
- Ondansetron (Oral, Oromucosal) (Advanced Reading)
Other brands
Professional resources
- Ondansetron monograph
- Ondansetron (FDA)
- Ondansetron Injection (FDA)
- Ondansetron ODT (FDA)
- Ondansetron Oral Solution (FDA)
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.


