I 115 Pill: white, capsule/oblong, 17mm
The pill with imprint I 115 (White, Capsule/Oblong, 17mm) has been identified as Lamivudine and Zidovudine 150 mg / 300 mg and is used for HIV Infection, Occupational Exposure, and Nonoccupational Exposure. It belongs to the drug class antiviral combinations and is not a controlled substance.
Images for I 115
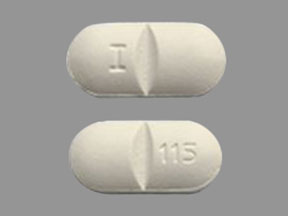

Lamivudine and Zidovudine
- Imprint
- I 115
- Strength
- 150 mg / 300 mg
- Color
- White
- Size
- 17.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- Antiviral combinations
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Camber Pharmaceuticals, Inc.
- National Drug Code (NDC)
- 31722-0739
- Inactive Ingredients
-
hypromelloses,
magnesium stearate,
microcrystalline cellulose,
polyethylene glycol,
polysorbate 80,
sodium starch glycolate type A potato,
titanium dioxide
Note: Inactive ingredients may vary.
Related images for "I 115"
More about lamivudine / zidovudine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (4)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: antiviral combinations
- En español
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.