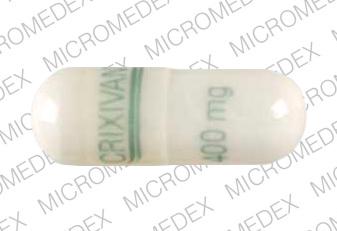
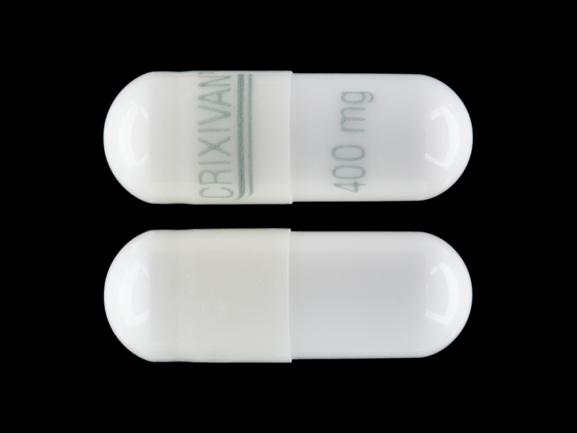
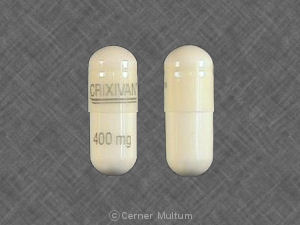
CRIXIVAN 400 mg Pill: white, capsule/oblong, 22mm
Generic Name: indinavir
The pill with imprint CRIXIVAN 400 mg (White, Capsule/Oblong, 22mm) has been identified as Crixivan 400 mg and is used for HIV Infection, Nonoccupational Exposure, and Occupational Exposure. It belongs to the drug class protease inhibitors and is not a controlled substance.
Images for CRIXIVAN 400 mg

Crixivan
- Generic Name
- indinavir
- Imprint
- CRIXIVAN 400 mg
- Strength
- 400 mg
- Color
- White
- Size
- 22.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- Protease inhibitors
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Merck & Company Inc.
- Inactive Ingredients
-
lactose anhydrous,
magnesium stearate,
titanium dioxide
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00006-0573 (Discontinued) | Merck |
| 35356-0139 (Discontinued) | Lake Erie Medical and Surgical Supply (repackager) |
| 52959-0507 (Discontinued) | H.J. Harkins Company, Inc. (repackager) |
| 54569-8620 (Discontinued) | A-S Medication Solutions, LLC (repackager) |
See also:
More about Crixivan (indinavir)
- Check interactions
- Compare alternatives
- Reviews (1)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: protease inhibitors
- Breastfeeding
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
