Omisirge Dosage
Generic name: OMIDUBICEL 8000000001 in 100mL; ; OMIDUBICEL NON-CULTURED FRACTION 4000000001 in 50mL
Dosage form: intravenous kit
Drug class: Miscellaneous uncategorized agents
Medically reviewed by Drugs.com. Last updated on Jan 10, 2025.
For intravenous use only.
Do not irradiate.
- Do NOT use a leukodepleting filter.
- Verify patient's identity upon receipt. Do NOT open the metal cassettes until time of thaw.
- Verify patient's identity prior to thaw and prior to infusion.
- Thawing should only take place immediately prior to use.
- Administration of OMISIRGE should be under the supervision of a physician experienced in treatment of hematologic malignancies, in centers with expertise in hematopoietic stem cell transplants.
Dose
A single dose of OMISIRGE consists of
- a Cultured Fraction (CF): a minimum of 8.0 × 108 total viable cells of which a minimum of 8.7% is CD34+ cells and a minimum of 9.2 × 107 CD34+ cells, and
- a Non-cultured Fraction (NF): a minimum of 4.0 × 108 total viable cells with a minimum of 2.4 × 107 CD3+ cells
The CF and NF are supplied cryopreserved separately in two bags. OMISIRGE requires thaw and dilution with two infusion solution (IS) bags (one IS bag for the CF, and one IS bag for the NF) prior to administration. Infusion of the NF bag should begin within 1 hour after completion of the CF infusion. For timing of dosing of each fraction, refer to section 2.2 under "Planning prior to OMISIRGE preparation".
Administration
The patient's identity must match the patient-specific identifiers on the CF and NF metal cassettes, the CF and NF bags, and the respective Infusion Solution for CF and NF bags. Do NOT infuse OMISIRGE if the information on the patient-specific labels does not match the intended patient.
Preparing the Patient for OMISIRGE Infusion
- Confirm the Released For Infusion Certificate (RFI Certificate) is available for OMISIRGE before starting the conditioning regimen.
- Administer an appropriate conditioning regimen before infusion of OMISIRGE, according to institutional guidelines.
- Administer prophylactic and supportive therapies for prevention or treatment of transplant complications (GvHD, infections) according to institutional guidelines.
Confirm emergency medications are available prior to infusion and during the recovery period as per institutional guidelines.
Premedication
- Premedicate the patient approximately 30 to 60 minutes prior to OMISIRGE infusion.
- Premedicate with diphenhydramine 50 mg IV (or 0.5 mg/kg up to a maximum of 50 mg) or dexchlorpheniramine 10 mg IV, hydrocortisone 50 mg IV (or 0.5 mg/kg up to a maximum of 50 mg) and acetaminophen 650 mg PO (or 10 mg/kg up to a maximum of 650 mg).
- Avoid prophylactic use of methylprednisolone in conjunction with OMISIRGE.
- Ensure the patient is adequately hydrated.
Receipt of OMISIRGE
OMISIRGE is shipped directly to the transplant center in 2 shipping containers:
- A liquid nitrogen dry vapor shipper containing the CF, the NF and a Chimerism Testing Sample(s) at ≤ - 150℃.
- A refrigerated shipping container containing the Infusion Solution for CF and the Infusion Solution for NF at 2-8℃.
- Confirm that the batch number and patient-specific identifiers on both shipping container labels match the intended patient and the information on the documents from the Gamida Cell Assist Hospital Portal.
- Confirm receipt of the Release for Shipping Certificate. Confirm patient-specific identifiers on the RFI Certificate and Certificates of Analysis (CoAs) match the patient's identity.
- Ensure that OMISIRGE was received in appropriate conditions and confirm that the temperature of the liquid nitrogen dry vapor shipper upon receipt was ≤ -150℃ and the temperature of the refrigerated shipping container was 2-8℃.
- If either of the shippers have expired upon arrival, or if you cannot confirm the patient identity with the patient-specific identifiers on any of the labels, contact Gamida Cell at (844) 477-7478.
- You should receive a total of 4 bags (i.e., CF Drug Product [DP] bag, NF DP bag, IS bag for CF DP and IS bag for NF DP) and vial or segment(s) containing Chimerism Testing Sample(s) in the OMISIRGE shipment.
The liquid nitrogen dry vapor shipper contains two metal cassettes, one labeled for the CF containing the CF cryopreserved bag and one labeled for the NF containing the NF cryopreserved bag. The shipper also contains a Chimerism Testing Sample(s).
- Do NOT open the metal cassettes until time of thaw since the product's overwrap bag may inflate preventing cassette closure.
- Verify that the products are within their expiry date by checking the label located on the front of the metal cassettes and through the cassette windows. Do NOT open the cassettes to locate the expiration date.
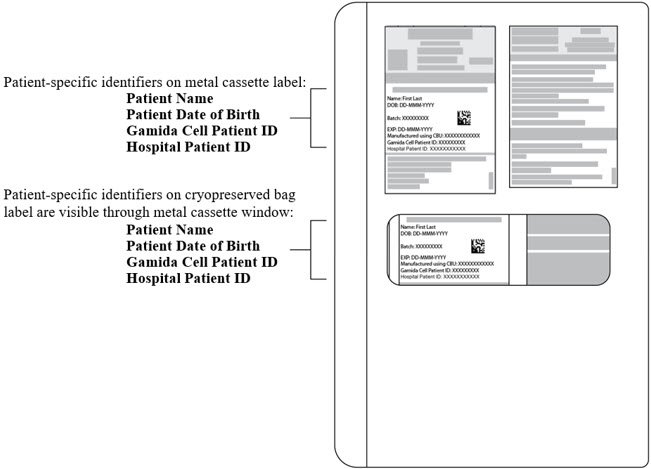
- Verify that the patient-specific identifiers on the labels on the outside of the CF and NF metal cassettes and on the CF and NF cryopreserved bags visible through the cassette window (see Figure 1) match the intended patient.
- Transfer the metal cassettes containing the CF and NF cryopreserved bags and the Chimerism Testing Sample(s) to onsite vapor phase of liquid nitrogen storage at ≤ -150℃.
Figure 1: CF or NF Cryopreserved Bag inside closed Metal Cassette.
Patient-specific identifiers are visible on the cryopreserved bag through the cassette window. Do NOT open the cassettes.
The refrigerated shipping container contains 2 IS bags, the IS for CF and the IS for NF, each with tubing and an attached spike adaptor. Each IS bag is packed inside a sterile bag.
- Ensure that both bags are intact and verify that the Infusion Solutions are within their expiry date by checking the expiration date on the labels located on the IS bags.
- Verify that the patient-specific identifiers on the IS bag labels match the intended patient (see Figure 2).
- Transfer both IS bags to refrigerated storage at 2-8°C.
Figure 2: Infusion Solution for CF Bag with Patient-Specific Label.
The IS bag has tubing with an attached spike adaptor and is packed inside a sterile bag.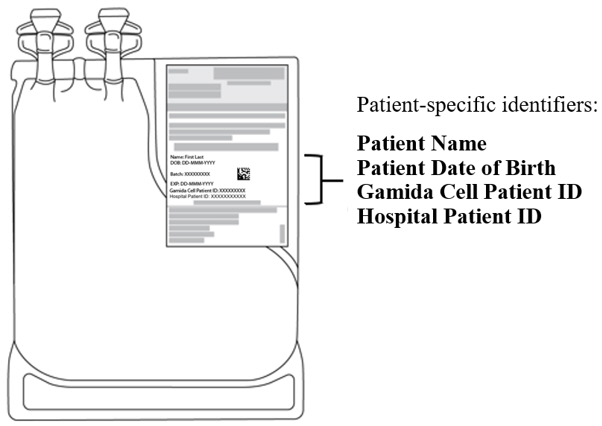
Planning prior to OMISIRGE preparation
- OMISIRGE must not be prepared until after receipt of the RFI Certificate for this patient-specific batch of OMISIRGE. CoAs for the CF, NF and IS batches are attached to the RFI Certificate. The RFI certificate will be issued via Gamida Cell Assist Hospital Portal up to 72 hours after completion of manufacturing.
- Confirm receipt of the RFI Certificate. Confirm patient-specific identifiers on the RFI Certificate and CoAs match the patient's identity.
- The CF bag must be administered FIRST.
- Confirm the infusion time in advance and adjust the start time of CF cryopreserved bag thaw so that it will be available for infusion when the patient is ready.
- Once the CF cryopreserved bag is removed from the metal cassette, thawing and dilution must be carried to completion and the cells administered within 2 hours post-dilution.
- Do not thaw the NF cryopreserved bag until you have determined that the CF has been safely administered.
- Once the NF cryopreserved bag is removed from the metal cassette, thawing and dilution must be carried to completion and the cells administered within 1 hour post-dilution.
- The infusion of the NF bag should begin within 1 hour after completion of the CF infusion.
Preparation of OMISIRGE for Infusion
- Follow universal precautions and local biosafety guidelines for handling and disposal of human cells to avoid potential transmission of infectious diseases.
- Use aseptic technique for all processing steps, including spiking of all transfusion infusion bag ports. No samples should be drawn from OMISIRGE.
The Cultured Fraction
Preparation of the Infusion Solution for CF
- 1.
- Remove the IS for CF bag from the 2-8°C storage location. Remove only the IS for CF bag at this time.
- 2.
- Confirm patient-specific identifiers on the label of the IS for CF match the intended patient.
- 3.
- Wipe the IS for CF sterile bag with 70% alcohol. Place it in the Biological Safety Cabinet (BSC) (if available), for at least 20 minutes with a maximum of 24 hours at room temperature.
- 4.
- Prior to dilution, remove the IS for CF bag from its sterile bag. Check that the pinch clamp is closed.
Thawing and diluting the CF
- 5.
- Remove the CF metal cassette from the liquid nitrogen storage.
- 6.
- Prior to opening the CF metal cassette, verify that the patient-specific identifiers on the label on the outside of the cassette and on the CF cryopreserved bag (visible through the cassette window) match the intended patient (see Figure 1).
- 7.
- Once patient identity has been verified, open the CF metal cassette to remove the CF cryopreserved bag from the cassette. Leave the CF cryopreserved bag in the overwrap bag during thawing and dilution.
- 8.
- Visually inspect the CF cryopreserved bag for damage. If the bag is damaged, contact Gamida Cell at (844) 477-7478. The cryopreserved CF should be white in color.
- 9.
- Once the CF cryopreserved bag is removed from the metal cassette, the thaw and dilution must be carried to completion and the cells administered within 2 hours post-dilution.
- 10.
- Incubate the CF cryopreserved bag for 5 minutes at room temperature.
- 11.
- Place the CF cryopreserved bag in an approximately 37°C water bath until the product reaches a liquid consistency. This generally takes about 3-8 minutes.
Do not massage, knead or apply pressure on the product bag. Keep the bag fully submerged until thawed – do not remove before thawing completion.
- 12.
- Remove the thawed bag from the water bath as soon as the cells have completely thawed. Do not remove the overwrap bag.
- 13.
- Wipe the overwrap with 70% alcohol. Put the bag into the BSC (if available).
- 14.
- Open the overwrap as follows:
- –
- Wipe a pair of clean scissors with 70% alcohol.
- –
- Cut the sealed area at the top of the overwrap.
Be careful not to damage the CF bag or the CF bag's ports/ tubing.
- 15.
- Insert the spike adapter attached to the IS for CF bag into one of the ports of the CF bag, while it remains in the overwrap bag.
- 16.
- Open the pinch clamp on the IS tubing and double the volume of the CF by adding IS for CF (approximately 20 mL) to the CF bag. Gently swirl the bag until mixed well.
- 17.
- Add the remaining IS for CF (approximately 60 mL) to the CF bag. Close the valve and swirl gently.
- 18.
- Remove the overwrap of the CF bag and check the integrity of the CF bag.
- 19.
- Check the appearance of the contents of the CF bag. The thawed and diluted CF should appear as a yellowish suspension, essentially free of visible white clumps and foreign particulates.
- 20.
- Inspect the contents of the thawed and diluted CF bag for any visible cell clumps. If visible cell clumps remain, gently invert and/or massage the bag with fingertips. Small clumps of cellular material should disperse with gentle manual mixing. Do not infuse the CF if clumps are not dispersed, the bag is damaged or leaking, or otherwise appears to be compromised. If this occurs, call Gamida Cell at (844) 477-7478.
- 21.
- Heat seal and detach the emptied Infusion Solution for CF bag.
- 22.
- Connect the Transfusion Infusion Set to the free port on the CF bag. Alternatively, the infusion set may be connected in accordance with internal procedures.
- 23.
- Place the CF bag containing the thawed and diluted CF in a new sterile bag.
Note: Do not wash, spin down, and/or resuspend CF in new media prior to infusion.
- 24.
- Transport the product to the patient at room temperature. Unless prepared at the patient's bedside, transport the product to the bedside in a closed box/bag to protect the product during transport.
The CF bag should be completely infused within 2 hours post-dilution. - 25.
- See the ‘OMISIRGE Administration’ section on how to infuse the CF.
The Non-cultured Fraction
Preparation of the Infusion Solution for NF
- 1.
- Remove the IS for NF bag from the 2-8°C storage location.
- 2.
- Repeat steps 2-4 from the CF process, for the IS for NF.
Thawing and diluting the NF
- 3.
- Repeat steps 5-8 from the CF process, for the NF. The cryopreserved NF should be red in color.
- 4.
- Once the NF cryopreserved bag is removed from the metal cassette, the thaw and dilution must be carried to completion and the cells administered within 1 hour post-dilution.
- 5.
- Repeat steps 10-14 from the CF process, for the NF.
- 6.
- Insert the spike adapter attached to the IS for NF bag into one of the ports of the NF bag, while it remains in the overwrap bag.
- 7.
- Open the pinch clamp on the IS tubing and double the volume of the NF by adding Infusion Solution for NF (approximately 10 mL) to the NF bag. Gently swirl the bag until mixed well.
- 8.
- Add the remaining IS for NF (approximately 30 mL) to the NF bag. Close the valve and swirl gently.
- 9.
- Repeat steps 18-21 from the CF process, for the NF. The thawed and diluted NF should appear as a reddish suspension essentially free of visible clumps and foreign particulates.
- 10.
- Repeat steps 22-25 from the CF process, for the NF. The NF should be completely infused within 1 hour post-dilution.
OMISIRGE Administration
Do NOT use a leukodepleting filter
- Central venous access is recommended for the infusion of OMISIRGE.
- Confirm that the patient's identity matches the patient-specific identifiers on the CF and NF bags.
- Administer OMISIRGE by gravity infusion.
- Prior to spiking both the CF and NF bags, prime the infusion set tubing with normal saline.
- Infuse the entire contents of the CF and NF bags.
- The rate of infusion should not exceed a maximum of 10 mL per kg per hour.
Administration:
- The thawed and diluted CF bag must be infused FIRST. The infusion time should not exceed 2 hours from the end of dilution to the end of CF infusion. Should an infusion reaction occur, appropriately manage the reaction before thawing the NF.
- The thawed and diluted NF is infused within 1 hour of safely administering the CF infusion. The infusion time should not exceed 1 hour from the end of dilution to the end of infusion.
- In the event of any deviation from the dosing schedule, contact Gamida Cell at (844) 477-7478.
- After the entire contents of the CF and NF bags are each infused, wash the tubing with normal saline at the same infusion rate to ensure as many cells as possible are delivered to the patient.
Follow universal precautions and local biosafety guidelines for handling and disposal of human cells to avoid potential transmission of infectious diseases.
Monitoring
- Monitor the patient for hypersensitivity or other infusion-related reactions during the infusion and post-infusion, per institutional guidelines.
- The infusion rate should be reduced if the fluid load is not tolerated. The infusion should be paused in the event of a hypersensitivity reaction or if the patient develops a moderate to severe infusion reaction. Appropriate medical therapy should be administered as needed.
- Monitor for graft failure, GvHD, infections and other post-transplant complications according to institutional guidelines.
More about Omisirge (omidubicel)
- Check interactions
- Compare alternatives
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous uncategorized agents
- En español
Patient resources
Professional resources
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.


