HyperRHO S/D Mini Dose Dosage
Generic name: Human Rho(d) Immune Globulin 250[iU] in 1mL
Dosage form: injection
Drug class: Immune globulins
Medically reviewed by Drugs.com. Last updated on Jun 24, 2025.
NEVER ADMINISTER HYPERRHO MINI-DOSE INTRAVENOUSLY. INJECT ONLY INTRAMUSCULARLY. ADMINISTER ONLY TO WOMEN POSTABORTION OR POSTMISCARRIAGE OF UP TO 12 WEEKS’ GESTATION. NEVER ADMINISTER TO THE NEONATE.
One syringe of HyperRHO Mini-Dose provides sufficient antibody to prevent Rh sensitization to 2.5 mL Rho(D) positive packed red cells or the equivalent (5 mL) of whole blood. This dose is sufficient to provide protection against maternal Rh sensitization for women undergoing spontaneous or induced abortion of up to 12 weeks’ gestation.
Rho(D) Immune Globulin (Human) — HyperRHO® Mini-Dose (250 IU; 50 mcg) should be administered within 3 hours or as soon as possible following spontaneous or induced abortion. If prompt administration is not possible, HyperRHO Mini-Dose should be given within 72 hours following termination of the pregnancy.
HyperRHO Mini-Dose is administered intramuscularly, preferably in the deltoid muscle of the upper arm or lateral thigh muscle. The gluteal region should not be used as an injection site because of the risk of injury to the sciatic nerve.(11)
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HyperRHO Mini-Dose is supplied with a syringe and an attached needle guard for your protection and convenience. Please follow instructions below for proper use of syringe and needle guard.
Directions for Syringe Usage
-
Remove the prefilled syringe from the package. Lift syringe by barrel, not by plunger.
-
Twist the plunger rod clockwise until the threads are seated. Do not use if the syringe is prematurely engaged.
-
With the rubber needle shield secured on the syringe tip, push the plunger rod forward a few millimeters to break any friction seal between the rubber stopper and the glass syringe barrel.
-
Remove the needle shield and expel air bubbles. [Do not remove the rubber needle shield to prepare the product for administration until immediately prior to the anticipated injection time.]
- Proceed with hypodermic needle puncture.
- Aspirate prior to injection to confirm that the needle is not in a vein or artery.
- Inject the medication.
-
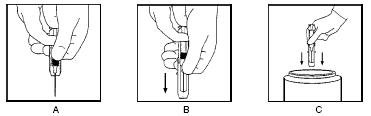
Keeping your hands behind the needle, grasp the guard with free hand and slide forward toward needle until it is completely covered and guard clicks into place. If audible click is not heard, guard may not be completely activated. (See Diagrams A and B)
-
Place entire prefilled glass syringe with guard activated into an approved sharps container for proper disposal. (See Diagram C)
A number of factors could reduce the efficacy of this product or even result in an ill effect following its use. These include improper storage and handling of the product after it leaves our hands, diagnosis, dosage, method of administration, and biological differences in individual patients. Because of these factors, it is important that this product be stored properly and that the directions be followed carefully during use.
More about HyperRHO S/D Mini-Dose (rho (d) immune globulin)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- Drug class: immune globulins
- Breastfeeding
- En español
Patient resources
Other brands
RhoGAM, Rhophylac, RhoGAM Ultra-Filtered Plus, WinRho SDF, ... +4 more
Professional resources
Other brands
Rhophylac, RhoGAM Ultra-Filtered Plus, HyperRHO S/D Full Dose
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.