HyperRHO S/D Mini Dose: Package Insert / Prescribing Info
Package insert / product label
Generic name: rho(d) immune globulin (human)
Dosage form: injection
Drug class: Immune globulins
J Code (medical billing code): J2792 (100 units, injection)
Medically reviewed by Drugs.com. Last updated on Jul 20, 2025.
On This Page
HyperRHO S/D Mini Dose Description
Rho(D) Immune Globulin (Human) — HyperRHO® Mini-Dose is a clear or slightly opalescent and colorless or pale yellow sterile solution of human rho(D) immune globulin containing antibodies to Rho(D) for intramuscular administration; it contains no preservative. HyperRHO Mini-Dose is prepared from pools of human plasma collected from healthy donors by a combination of cold ethanol fractionation, caprylate precipitation and filtration, caprylate incubation, anion exchange chromatography, nanofiltration and low pH incubation. HyperRHO Mini-Dose is formulated as a 15% to 18% protein solution at a pH of 4.1 to 4.8 in 0.16 M to 0.26 M glycine. One dose of HyperRHO Mini-Dose contains not less than one-sixth the quantity of Rho(D) antibody contained in one standard dose of Rho(D) Immune Globulin (Human), and it will suppress the immunizing potential of 2.5 mL of Rho(D) positive packed red blood cells or the equivalent of whole blood (5 mL). The quantity of Rho(D) antibody in HyperRHO Mini-Dose is not less than 250 IU (50 mcg) per 1 mL.
When medicinal biological products are administered, the risk of infectious diseases due to transmission of pathogens cannot be totally excluded. However, in the case of products prepared from human plasma, the risk of transmission of pathogens is reduced by epidemiological surveillance of the donor population and selection of individual donors by medical interview; testing of individual donations and plasma pools; and the presence in the manufacturing processes of steps with demonstrated capacity to inactivate/remove pathogen.
In the manufacturing process of HyperRHO Mini-Dose, there are several steps with the capacity for viral inactivation or removal.(1) The main steps of the manufacturing process that contribute to the virus clearance capacity are as follows:
- Caprylate precipitation/depth filtration
- Caprylate incubation
- Depth filtration
- Column chromatography
- Nanofiltration
- Low pH final container incubation
To provide additional assurance of the pathogen safety of the final product, the capacity of the HyperRHO Mini-Dose manufacturing process to remove and/or inactivate viruses has been demonstrated by laboratory spiking studies on a scaled down process model using a wide range of viruses with diverse physicochemical properties.
The combination of all of the above-mentioned measures provides the final product with a high margin of safety from the potential risk of transmission of infectious viruses.
The caprylate/chromatography manufacturing process was also investigated for its capacity to decrease the infectivity of an experimental agent of transmissible spongiform encephalopathy (TSE), considered as a model for the variant Creutzfeldt-Jakob disease (vCJD) and Creutzfeldt-Jakob disease (CJD) agents.(1) These studies provide reasonable assurance that low levels of CJD/vCJD agent infectivity, if present in the starting material, would be removed by the caprylate/chromatography manufacturing process.
HyperRHO S/D Mini Dose - Clinical Pharmacology
Rh sensitization may occur in nonsensitized Rho(D) negative women following transplacental hemorrhage resulting from spontaneous or induced abortions.(2,3) The risk of sensitization is higher in women undergoing induced abortions than in those aborting spontaneously.(3-4)
HyperRHO Mini-Dose is used to prevent the formation of anti-Rho(D) antibody in Rho(D) negative women who are exposed to the Rho(D) antigen at the time of spontaneous or induced abortion (up to 12 weeks’ gestation).(4-6) HyperRHO Mini-Dose suppresses the stimulation of active immunity by Rho(D) positive fetal erythrocytes that may enter the maternal circulation at the time of termination of the pregnancy.
The amount of anti-Rho(D) in HyperRHO Mini-Dose has been shown to effectively prevent maternal isosensitization to the Rho(D) antigens following spontaneous or induced abortion occurring up to the 12th week of gestation.(7-9) After the 12th week of gestation, a standard dose of HyperRHO Full Dose is indicated.
In a clinical study in 12 healthy human adults receiving another hyperimmune immune globulin product, Rabies Immune Globulin (Human), HyperRAB®, prepared by the same manufacturing process, detectable passive antibody titers were observed in the serum of all subjects by 24 hours post injection and persisted through the 21 day study period.
Indications and Usage for HyperRHO S/D Mini Dose
HyperRHO Mini-Dose is recommended to prevent the isoimmunization of Rho(D) negative women at the time of spontaneous or induced abortion of up to 12 weeks’ gestation provided the following criteria are met:
-
The mother must be Rho(D) negative and must not already be sensitized to the Rho(D) antigen.
-
The father is not known to be Rho(D) negative.
-
Gestation is not more than 12 weeks at termination.
Note: Rho(D) Immune Globulin (Human) prophylaxis is not indicated if the fetus or father can be determined to be Rh negative. If the Rh status of the fetus is unknown, the fetus must be assumed to be Rho(D) positive, and HyperRHO Mini-Dose should be administered to the mother.
FOR ABORTIONS OR MISCARRIAGES OCCURRING AFTER 12 WEEKS’ GESTATION, A STANDARD DOSE OF Rho(D) IMMUNE GLOBULIN (HUMAN) IS INDICATED.
HyperRHO Mini-Dose should be administered within 3 hours or as soon as possible after spontaneous passage or surgical removal of the products of conception. However, if HyperRHO Mini-Dose is not given within this time period, consideration should still be given to its administration since clinical studies in male volunteers have demonstrated the effectiveness of Rho(D) Immune Globulin (Human) in preventing isoimmunization as long as 72 hours after infusion of Rho(D) positive red cells.(10)
Warnings
HyperRHO Mini-Dose is made from human plasma. Products made from human plasma may contain infectious agents, such as viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob Disease (CJD) agent that can cause disease. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by including manufacturing steps that inactivate and/or remove viruses. Despite these measures, such products can still potentially transmit disease. There is also the possibility that unknown infectious agents may be present in such products. Individuals who receive infusions of blood or plasma products may develop signs and/or symptoms of some viral infections, particularly hepatitis C. ALL infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Grifols Therapeutics LLC [1-800-520-2807].
The physician should discuss the risks and benefits of this product with the patient, before prescribing or administering it to the patient.
NEVER ADMINISTER HYPERRHO MINI-DOSE INTRAVENOUSLY. INJECT ONLY INTRAMUSCULARLY. ADMINISTER ONLY TO WOMEN POSTABORTION OR POSTMISCARRIAGE OF UP TO 12 WEEKS’ GESTATION. NEVER ADMINISTER TO THE NEONATE.
HyperRHO Mini-Dose should be given with caution to patients with a history of prior systemic allergic reactions following the administration of human immune globulin preparations.
The attending physician who wishes to administer HyperRHO Mini-Dose to persons with isolated immunoglobulin A (IgA) deficiency must weigh the benefits of immunization against the potential risks of hypersensitivity reactions. Such persons have increased potential for developing antibodies to IgA and could have anaphylactic reactions to subsequent administration of blood products that contain IgA.
As with all preparations administered by the intramuscular route, bleeding complications may be encountered in patients with thrombocytopenia or other bleeding disorders.
Precautions
General
Although systemic reactions to immunoglobulin preparations are rare, epinephrine should be available for treatment of acute anaphylactic symptoms.
Drug Interactions
Other antibodies in the HyperRHO Mini-Dose preparation may interfere with the response to live vaccines such as measles, mumps, polio or rubella. Therefore, immunization with live vaccines should not be given within 3 months after HyperRHO Mini-Dose administration.
Pregnancy
Animal reproduction studies have not been conducted with HyperRHO Mini-Dose. It is also not known whether HyperRHO Mini-Dose can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity.
It should be again noted, however, that HyperRHO Mini-Dose is not indicated for use during pregnancy and it should be administered only postabortion or postmiscarriage.
Adverse Reactions/Side Effects
Reactions to HyperRHO Mini-Dose are infrequent in Rho(D) negative individuals and consist primarily of slight soreness at the site of injection and slight temperature elevation. While sensitization to repeated injections of human globulin is extremely rare, it has occurred.
Related/similar drugs
HyperRHO S/D Mini Dose Dosage and Administration
NEVER ADMINISTER HYPERRHO MINI-DOSE INTRAVENOUSLY. INJECT ONLY INTRAMUSCULARLY. ADMINISTER ONLY TO WOMEN POSTABORTION OR POSTMISCARRIAGE OF UP TO 12 WEEKS’ GESTATION. NEVER ADMINISTER TO THE NEONATE.
One syringe of HyperRHO Mini-Dose provides sufficient antibody to prevent Rh sensitization to 2.5 mL Rho(D) positive packed red cells or the equivalent (5 mL) of whole blood. This dose is sufficient to provide protection against maternal Rh sensitization for women undergoing spontaneous or induced abortion of up to 12 weeks’ gestation.
Rho(D) Immune Globulin (Human) — HyperRHO® Mini-Dose (250 IU; 50 mcg) should be administered within 3 hours or as soon as possible following spontaneous or induced abortion. If prompt administration is not possible, HyperRHO Mini-Dose should be given within 72 hours following termination of the pregnancy.
HyperRHO Mini-Dose is administered intramuscularly, preferably in the deltoid muscle of the upper arm or lateral thigh muscle. The gluteal region should not be used as an injection site because of the risk of injury to the sciatic nerve.(11)
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
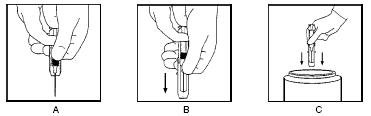
HyperRHO Mini-Dose is supplied with a syringe and an attached needle guard for your protection and convenience. Please follow instructions below for proper use of syringe and needle guard.
Directions for Syringe Usage
-
Remove the prefilled syringe from the package. Lift syringe by barrel, not by plunger.
-
Twist the plunger rod clockwise until the threads are seated. Do not use if the syringe is prematurely engaged.
-
With the rubber needle shield secured on the syringe tip, push the plunger rod forward a few millimeters to break any friction seal between the rubber stopper and the glass syringe barrel.
-
Remove the needle shield and expel air bubbles. [Do not remove the rubber needle shield to prepare the product for administration until immediately prior to the anticipated injection time.]
- Proceed with hypodermic needle puncture.
- Aspirate prior to injection to confirm that the needle is not in a vein or artery.
- Inject the medication.
-
Keeping your hands behind the needle, grasp the guard with free hand and slide forward toward needle until it is completely covered and guard clicks into place. If audible click is not heard, guard may not be completely activated. (See Diagrams A and B)
-
Place entire prefilled glass syringe with guard activated into an approved sharps container for proper disposal. (See Diagram C)
A number of factors could reduce the efficacy of this product or even result in an ill effect following its use. These include improper storage and handling of the product after it leaves our hands, diagnosis, dosage, method of administration, and biological differences in individual patients. Because of these factors, it is important that this product be stored properly and that the directions be followed carefully during use.
How is HyperRHO S/D Mini Dose supplied
HyperRHO Mini-Dose is available in a single-dose syringe with attached needle. The HyperRHO Mini-Dose package contains 10 single-dose syringes. HyperRHO Mini-Dose contains no preservative and is not made with natural rubber latex.
| NDC Number | Size |
| 13533-661-06 | Syringe (10 pack) |
Storage and Handling
Store at 2°C to 8°C (36°F to 46°F). Do not freeze. Do not use after expiration date. Discard unused portion.
References
-
Barnette D, Roth NJ, Hotta J, et al. Pathogen safety profile of a 10% IgG preparation manufactured using a depth filtration-modified process. Biologicals 2012;40:247-53.
- Queenan JT, Shah S, Kubarych SF, et al. Role of induced abortion in rhesus immunisation. Lancet 1971;1(7704):815-7.
- Goldman JA, Eckerling B. Prevention of Rh immunization after abortion with anti-Rho(D)-immunoglobulin. Obstet Gynecol 1972;40(3):366-70.
- The selective use of Rho(D) immune globulin (RhIG). ACOG Tech Bull 61,1981.
- Prevention of Rh sensitization. WHO Tech Rep Ser 468, 1971.
- Recommendation of the Public Health Service Advisory Committee on Immunization Practices. Rh immune globulin. MMWR 1972;21(15):126-7.
- Stewart FH, Burnhill MS, Bozorgi N. Reduced dose of Rh immunoglobulin following first trimester pregnancy termination. Obstet Gynecol 1978;51(3):318-22.
- McMaster conference on prevention of Rh immunization. 28-30 September, 1977. Vox Sang 1979;36(1):50-64.
- Simonovits I. Efficiency of anti-D IgG prevention after induced abortion. Vox Sang 1974;26(4):361-7.
- Freda VJ, Gorman JG, Pollack W Prevention of Rh-hemolytic disease with Rh-immune globulin. Am J Obstet Gynecol 1977;128(4):456-60.
- Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP). General recommendations on immunization. MMWR 2002;51(RR02):1-36.
(Rev. 5/2025)
GRIFOLS
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA 3068045
U.S. License No. 1871
Patient Counseling Information
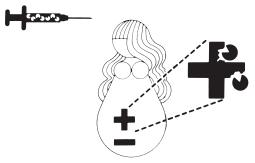
The Rh Factor and Your Pregnancy

Information About Pregnancy Protection
The Rh Factor and When It Is Important
The Rh factor is one of many blood group antigens found on the surface of red blood cells. If you have this antigen you are considered Rh positive. If you don’t, then you are considered Rh negative. Everyone is either Rh positive or Rh negative. One type is neither better nor worse than the other, only different.
Your Rh factor is important if you are an Rh negative woman and you become pregnant, or if you receive a blood transfusion.
How the Rh Factor Can Affect Your Future
If you have Rh negative blood, there are two situations that can affect you:
- 1.
- If the father of your baby is Rh positive, the baby will probably be Rh positive too. An Rh negative woman carrying an Rh positive baby may have an immune reaction if some of the baby’s Rh positive blood cells enter her bloodstream.
-
This immune reaction, called isoimmunization, means your body’s defense system recognizes Rh positive blood as foreign from your own and produces “antibodies” to destroy the invading Rh positive blood cells. -
The passage of blood from the baby to the mother’s bloodstream happens most often at delivery, but can also occur during miscarriage, the termination of pregnancy, amniocentesis (test performed to determine fetal health), or due to an injury or trauma. It is important to note that a small number of women develop antibodies to Rh positive blood cells during pregnancy for no apparent reason. -
Antibodies to Rh positive blood may not be a problem in first pregnancies; however, the antibodies stay in your bloodstream, ready to attack invading Rh positive blood cells, for many years to come. This can lead to problems in future pregnancies by causing miscarriage or a disease known as hemolytic disease of the newborn. -
Babies born to Rh positive mothers, regardless of the father’s blood type, will usually be free of the dangers of hemolytic disease.
- 2.
- Someday it may become necessary for you to receive a blood transfusion. If Rh positive antibodies already reside in your bloodstream due to isoimmunization and the blood you receive is Rh positive due to error or lifesaving reasons, your Rh positive antibodies will become mobilized and destroy the donor Rh positive cells. As a result, the transfusion could be unsuccessful and possibly harmful to you.
Hemolytic Disease of the Newborn: A Threat to Your Baby
When an Rh negative woman has Rh positive antibodies in her blood and the baby she is carrying is Rh positive, the antibodies could possibly enter the baby’s bloodstream, attack the baby’s red blood cells and cause hemolytic disease of the newborn. At birth, the infant suffering from hemolytic disease may be jaundiced and anemic or suffer permanent damage of the brain and central nervous system which may result in mental retardation, hearing loss, or cerebral palsy. Extensive medical care can be required, including an exchange transfusion, in which all of the baby’s blood is replaced. This usually stops the destruction of the baby’s red blood cells and gives the infant a chance to survive.
The risk of hemolytic disease of the newborn is slight with the first baby, but increases with each successive pregnancy.
Preventing Hemolytic Disease
HyperRHO® , Rho(D) Immune Globulin (Human), can prevent hemolytic disease of the newborn, provided Rh positive antibodies do not already reside in your bloodstream.
HyperRHO is a specially prepared gamma globulin with a high level of preformed antibodies against Rh positive blood cells. The injection of HyperRHO destroys any Rh positive blood cells that may have entered the mother’s bloodstream and prevents the mother’s immune system from producing Rh positive antibodies; thus protecting the baby from developing hemolytic disease.
HyperRHO Full Dose — When Prescribed
Pregnancy and Other Obstetric Conditions Pertaining to Rh Negative Women
HyperRHO Full Dose (1500 IU; 300 mcg) is administered during pregnancy if you fall into a high-risk category. For example, you are at risk of producing Rh positive antibodies if you have an amniocentesis procedure performed, or if you have a miscarriage or other termination of pregnancy at or beyond 13 weeks' gestation.
Laboratory findings have shown that some Rh negative women develop Rh positive antibodies during the last weeks of pregnancy even without an antibody-stimulating event. As a preventive measure, your physician will probably recommend the first injection of HyperRHO Full Dose at the 28th week of pregnancy.
In both of the above situations, if the blood type of the father or baby can be determined to be Rh negative, an injection of HyperRHO is not required.
Another injection of HyperRHO Full Dose is administered within 72 hours of delivery of an Rh positive baby.
Blood Transfusion
HyperRHO Full Dose (1500 IU; 300 mcg) may be used to prevent isoimmunization in Rh negative individuals who have been transfused with Rh positive red blood cells or blood components containing red blood cells.
HyperRHO Mini-Dose — When Prescribed
A single dose of HyperRHO Mini-Dose (250 IU; 50 mcg) may be prescribed for an Rh negative woman instead of HyperRHO Full Dose (1500 IU; 300 mcg) in the event of miscarriage or other termination of pregnancy occurring prior to 13 weeks' gestation. HyperRHO Mini-Dose is not required if the blood type of the father or fetus can be determined to be Rh negative.
Will You Need HyperRHO Again?
HyperRHO provides protection only if you have not already produced Rh positive antibodies. Women who have developed antibodies through previous pregnancy, miscarriage, other termination of pregnancy, or blood transfusion cannot be protected by HyperRHO. This is why with each pregnancy it is important to have HyperRHO injections within the prescribed time period.
Reactions to HyperRHO
You may feel a temporary soreness at the site of the injection. You may also have a slight and temporary change in body temperature. In very rare instances, an allergic type of reaction can occur, for which your physician will take appropriate measures.
Delivering a Sound, Healthy Baby
Your physician can answer any questions you may have about receiving a HyperRHO injection to prevent hemolytic disease of the newborn. If you know that you are Rh negative and you are pregnant, you should discuss your situation with your physician. Today, with HyperRHO, hemolytic disease of the newborn can be reduced to its lowest possible rate of incidence.
GRIFOLS
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA 3068045
U.S. License No. 1871 (Rev. 5/2025)
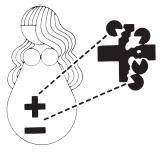
Development of Hemolytic Disease
| 1 Rh positive (+) father. Rh negative (–) mother.
| 2 Pregnancy: Rh– mother is carrying Rh+ baby.
|
| 3 The passage of Rh+ blood from the baby to the mother’s bloodstream happens most often at delivery, but can also occur during miscarriage, other termination of pregnancy, amniocentesis, or due to injury or trauma.
| 4 Rh+ antibodies stay in your bloodstream, ready to attack invading Rh+ blood cells, for many years to come.
|
| 5 Next pregnancy, mother’s Rh+ antibodies enter baby’s Rh+ bloodstream, attacking baby’s blood cells and causing hemolytic disease of the newborn.
|
How HyperRHO Immune Globulin Can Prevent Hemolytic Disease
| 1 You will probably be given two injections of HyperRHO Full Dose, one at the 28th week of your pregnancy and another within 72 hours of delivery, miscarriage or other termination of pregnancy. A single injection of HyperRHO Mini-Dose may be prescribed instead of HyperRHO Full Dose in the event of miscarriage or other termination of pregnancy occurring prior to 13 weeks' gestation.
| 2 HyperRHO immunization prevents formation of mother’s own Rh+ antibodies. Mother’s bloodstream remains free of Rh+ antibodies.
|
| 3 Next pregnancy, baby develops normally. HyperRHO should be administered following delivery, miscarriage, or other termination of pregnancy to continue protection if baby is Rh+.
|

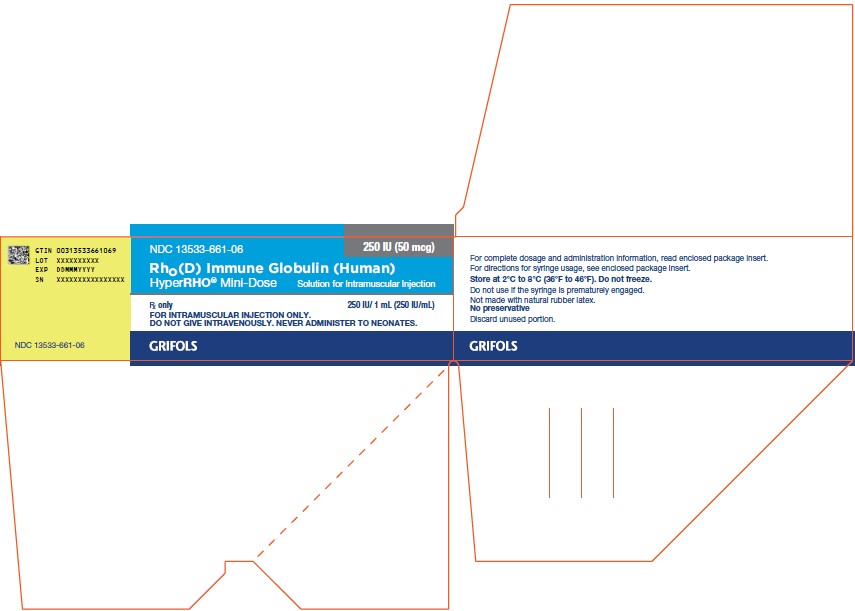
PACKAGE LABEL
NDC 13533-661-06
250 IU (50 mcg)
Rho(D) Immune Globulin (Human)
HyperRHO® Mini-Dose
Solution for Intramuscular Injection
250 IU / 1 mL (250 IU/mL)
Rx only
FOR INTRAMUSCULAR INJECTION ONLY.
DO NOT GIVE INTRAVENOUSLY. NEVER ADMINISTER TO NEONATES.
GRIFOLS
Contents: 10 single-dose disposable syringes with attached needles
Grifols Therapeutics LLC
Research Triangle Park,
NC 27709 USA
U.S. License No. 1871
GRIFOLS
NDC 13533-661-06
250 IU (50 mcg)
Rho(D) Immune Globulin (Human)
HyperRHO® Mini-Dose
10 x 1 mL
Solution for Intramuscular Injection
250 IU / 1 mL (250 IU/mL)
Contents: 10 single dose disposable syringes with attached needles
Rho(D) Immune Globulin (Human) - HyperRHO® Mini-Dose is a sterile solution of immunoglobulin containing
15% to 18% protein stabilized with 0.16 M to 0.26 M glycine.
The quantity of Rho(D) antibody in each single-dose syringe of HyperRHO® Mini-Dose is not less than
250 IU (50 mcg). One dose will suppress the immunizing potential of 2.5 mL Rho(D) positive packed red cells or the equivalent of whole blood (5.0 mL).
The patient and physician should discuss the risks and benefits of this product.
Rx only
FOR INTRAMUSCULAR INJECTION ONLY.
DO NOT GIVE INTRAVENOUSLY. NEVER ADMINISTER TO NEONATES.

NDC 13533-661-06
250 IU (50 mcg)
Rho(D) Immune Globulin (Human)
HyperRHO® Mini-Dose
Solution for Intramuscular Injection
250 IU / 1 mL (250 IU/mL)
Rx only
FOR INTRAMUSCULAR INJECTION ONLY.
DO NOT GIVE INTRAVENOUSLY. NEVER ADMINISTER TO NEONATES.
GRIFOLS
For complete dosage and administration information, read enclosed package insert.
For directions for syringe usage, see enclosed package insert.
Store at 2°C to 8°C (36°F to 46°F). Do not freeze.
Do not use if the syringe is prematurely engaged.
Not made with natural rubber latex.
No preservative
Discard unused portion.
GRIFOLS
GTIN 00313533661069
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXXXXXXXX
NDC 13533-661-06
3066813
Lot
Exp.
Rho(D) Immune
Globulin (Human)
HyperRHO® Mini-Dose
The patient and physician should discuss
the risks and benefits of this product.
Grifols Therapeutics LLC
RTP, NC 27709 USA
U.S. License No. 1871
250 IU

| HYPERRHO
MINI-DOSE
rho(d) immune globulin (human) solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - GRIFOLS USA, LLC (048987452) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GRIFOLS THERAPEUTICS LLC | 611019113 | manufacture(13533-661) | |
More about HyperRHO S/D Mini-Dose (rho (d) immune globulin)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- Dosage information
- During pregnancy
- Drug class: immune globulins
- Breastfeeding
- En español
Patient resources
Professional resources
Other brands
Rhophylac, RhoGAM Ultra-Filtered Plus, HyperRHO S/D Full Dose