Skytrofa
Pronunciation: sky-trow-fa
Generic name: lonapegsomatropin-tcgd
Dosage form: injection for subcutaneous use (0.7 mg, 1.4 mg, 1.8 mg, 2.1 mg, 2.5 mg, 3 mg, 3.6 mg, 4.3 mg, 5.2 mg, 6.3 mg, 7.6 mg, 9.1 mg, 11 mg, 13.3 mg)
Drug class: Growth hormones
What is Skytrofa?
Skytrofa is a human growth hormone used to treat growth failure due to inadequate secretion of endogenous growth hormone (GH) in children 1 year and older who weigh at least 11.5 kg (25.3 lb). Skytrofa is also used to replace endogenous growth hormone in adults with growth hormone deficiency (GHD). It is given as a subcutaneous injection once a week.
Skytrofa is a long-acting prodrug that converts to somatropin (synthetic growth hormone) in the body. It consists of unmodified somatropin temporarily attached to a polyethylene glycol carrier, which shields the hormone from kidney clearance, extending its half-life to about 25 hours for once-weekly dosing. Once released, the somatropin binds to growth hormone receptors, stimulating normal growth and development in children with growth hormone deficiency. In adults with growth hormone deficiency, it promotes protein synthesis, glucose production, and fat breakdown and can improve body composition by reducing trunk fat, increasing lean muscle mass, and normalizing metabolism.
Skytrofa first gained FDA approval on August 25, 2021. Approval was extended on July 28, 2025, to include adults with GHD. There is no generic.
- Approval in children was based on the Phase 3 heiGHt clinical trial (NCT02781727, n=161 treatment-naïve children with GHD), which demonstrated that once-weekly Skytrofa was superior to daily somatropin with a significantly higher annualized height velocity at 52 weeks (11.2 vs 10.3 cm/year) and greater height improvement, with comparable safety profiles between treatments.
Side effects
Common Skytrofa side effects:
The most common side effects of Skytrofa in children are:
- viral infections, such as a cold
- fever
- cough
- nausea and vomiting
- bleeding problems such as nosebleeds, pinpoint purple or red spots under your skin
- diarrhea
- stomach pain
- joint pain and arthritis.
The most common side effects of Skytrofa in adults are:
- fluid retention and swelling in the extremities, particularly the legs, ankles, and feet.
Serious side effects
Get emergency medical help if you have signs of an allergic reaction, such as hives, difficulty breathing, or swelling of your face, lips, tongue, or throat.
Skytrofa may cause other serious side effects. Call your doctor at once if you have:
- pain in your knees or hips, walking with a limp;
- numbness or tingling in your wrist, hand, or fingers;
- severe swelling or puffiness in your hands and feet;
- changes in behavior;
- vision problems, unusual headaches;
- changes in the shape or size of a mole;
- pain or swelling in your joints;
- pancreatitis - severe pain in your upper stomach spreading to your back, nausea and vomiting;
- high blood sugar - increased thirst, increased urination, dry mouth, fruity breath odor;
- increased pressure inside the skull--severe headaches, ringing in your ears, dizziness, nausea, vision problems, pain behind your eyes; or
- signs of an adrenal gland problem--extreme weakness, severe dizziness, weight loss, changes in skin color, feeling very weak or tired.
This is not a complete list of side effects, and others may occur. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
Related/similar drugs
Warnings
Call your doctor at once if you have signs of an allergic reaction to Skytrofa, severe pain in your upper stomach spreading to your back, increased thirst, increased urination, severe headaches, ringing in your ears, dizziness, nausea, vision problems, weight loss, changes in skin color, or feel very weak or tired.
Before taking this medicine
Do not use Skytrofa if you are allergic to it or if you have:
- hypersensitivity to somatropin, Skytrofa, or any of its ingredients
- acute critical illness after open heart surgery, abdominal surgery, or multiple accidental trauma
- acute respiratory failure
- active cancer
- eye problems caused by diabetes (diabetic retinopathy)
- Prader-Willi syndrome, and you are overweight or have severe breathing problems (including sleep apnea.
Tell your doctor if you have ever had:
- cancer (especially during childhood)
- diabetes
- a head injury or brain tumor
- childhood brain cancer and radiation treatment
- a pituitary gland disorder
- underactive thyroid
- abnormal curvature of the spine (scoliosis)
- breathing problems, sleep apnea (breathing stops during sleep).
Tell your doctor if you are pregnant or breastfeeding.
How is Skytrofa given?
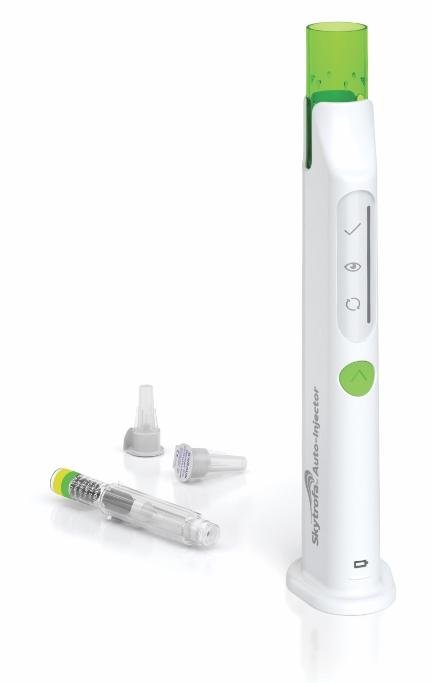
Skytrofa injection is given once a week under the skin of the abdomen, thigh, or buttocks using the Skytrofa autoinjector.
- It can be injected under the skin of the abdomen, thigh, or buttocks.
- Rotate injection sites between and within regions to reduce the risk of lipoatrophy.
- Do not inject into scar tissue, bruises, damaged skin, or in the same place twice in a row.
- The Skytrofa Auto-Injector is an electronic, reusable device that can be used at home by the patient or caregiver.
A healthcare provider can teach you how to properly use the Skytrofa auto-injector by yourself or for your child.
If the injection has been kept refrigerated, remove it from the fridge and keep it at room temperature for 15 minutes before use.
- Prepare an injection only when you are ready to give it.
- The Skytrofa Auto-Injector must be used with the Skytrofa cartridge. The cartridge has 2 chambers, 1 filled with powder and 1 filled with water. The auto-injector automatically mixes the powder and the water during preparation, making it ready for injection. Use the cartridges within 4 hours of inserting them into the autoinjector.
- The mixed solution should be clear and colorless to opalescent and may occasionally contain air bubbles. You should NOT inject if the solution is cloudy or contains particulate matter.
- When the injection needle is inserted into the skin, the device automatically delivers the drug product. The built-in electronics and software assist the user during the entire preparation and injection sequence and provide confirmation that the full dose has been delivered.
Do not reuse a needle or syringe. Place them in a puncture-proof "sharps" container and dispose of it following state or local laws. Keep out of the reach of children and pets.
Never share an injection pen, cartridge, or syringe, even if you changed the needle. Sharing these devices can pass infections from person to person.
Dosing information
Dose of Skytrofa for Children with Inadequate GH Secretion:
- Initial: 0.24 mg/kg body weight, given once weekly.
- Individualize and titrate the dosage based on response.
- Changing from daily somatropin therapy to once-weekly Skytrofa: Wait at least 8 hours between the final dose of daily somatropin and the first dose of once-weekly Skytrofa.
Comments: If patients experience failure to increase height velocity, particularly during the first year of treatment, assess compliance and evaluate other causes of poor growth, such as hypothyroidism, undernutrition, advanced bone age, and antibodies to recombinant human growth hormone.
Children who were treated with Skytrofa for GH deficiency in childhood and whose epiphyses are closed should be reevaluated before continuing with Skytropha.
Dose of Skytrofa for Adults with Growth Hormone Deficiency (GHD):
The initial dose of Skytrofa for adults with GHD is based on age and use of oral estrogen.
- Adults 30 to 60 years old (no oral estrogen intake): 1.4 mg once weekly.
- Adults under 30 or taking estrogen (any age): 2.1 mg once weekly
- Adults over 60 years (no oral estrogen intake): 0.7 mg once weekly.
Titration:
- Increase the dose monthly to a higher strength cartridge based on the clinical response and/or IGF-1 concentration. Draw IGF-1 serum sample 4 to 5 days after the prior dose.
- Decrease the dose to a lower strength cartridge as needed based on adverse reactions or a weekly average IGF-1 concentration above the age- and sex-specific normal range.
Maximum:
- The maximum recommended dose is 6.3 mg once weekly.
Comments:
- When changing from daily somatropin therapy to once-weekly Skytrofa, wait at least 8 hours between the final dose of daily somatropin and the first dose of Skytrofa.
- When changing from another once-weekly growth hormone therapy to once-weekly Skytrofa, wait at least 7 days between the final dose of the previous growth hormone therapy and the first dose of Skytrofa.
What happens if I miss a dose?
Use the medicine as soon as you can, but skip the missed dose if you are more than 2 days late for the dose. Do not use two doses at one time.
What happens if I overdose?
Seek emergency medical attention or call the Poison Help line at 1-800-222-1222.
An overdose can cause tremors or shaking, cold sweats, increased hunger, headache, drowsiness, weakness, dizziness, fast heartbeat, and nausea. Long-term overdose may cause excessive growth.
What should I avoid while using Skytrofa?
Follow your doctor's instructions about any restrictions on food, beverages, or activity.
What other drugs will affect Skytrofa?
Sometimes it is not safe to use certain medicines at the same time. Some drugs can affect your blood levels of other drugs you use, which may increase side effects or make the medicines less effective.
Tell your doctor about all your other medicines, especially:
- birth control pills or hormone replacement therapy
- insulin or oral diabetes medicine
- a steroid (prednisone, dexamethasone, methylprednisolone, and others)
- Certain medications metabolized by the cytochrome P450 system.
This list is not complete. Other drugs may affect Skytrofa, including prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible drug interactions are listed here. See the Drugs.com Drug Interaction Checker to find potentially harmful drug, food, and alcohol interactions.
Storage
Fridge storage
Refrigerate cartridges at 36°F to 46°F (2°C to 8°C). Do not freeze
- Keep cartridges in the outer carton to protect them from light.
- Skytrofa may be used until the expiration date when stored in a refrigerator.
Room temperature storage
Outer cartons containing blistered cartridges may be stored at room temperature [up to 86°F (30°C)] for up to 6 months and can be returned to refrigeration within 6 months. Write the date first removed from the refrigerator in the space provided on the outer carton.
- Do not use Skytrofa beyond the expiration date or 6 months after the date it was first removed from refrigeration (whichever is earlier).
Skytrofa ingredients
Active ingredient: lonapegsomatropin-tcgd
Inactive ingredient: water for injection.
Skytrofa is a white to off-white lyophilized powder available in a single-dose, dual-chamber, prefilled
cartridge containing lonapegsomatropin-tcgd in one chamber and diluent, Water for Injection, in the other
chamber and is available in the following strengths for injection: 0.7 mg, 1.4 mg, 1.8 mg, 2.1 mg, 2.5 mg, 3 mg, 3.6 mg, 4.3 mg, 5.2 mg, 6.3 mg, 7.6 mg, 9.1 mg, 11 mg, and 13.3 mg.
Company
Skytrofa is manufactured by Ascendis Pharma Endocrinology Division A/S, Tuborg Boulevard 12, Hellerup, Denmark DK-2900.
Skytrofa Biosimilars
Biosimilar and interchangeable products are biological products that are highly similar to and have no clinically meaningful differences from the reference product.
Reference products
These are biological products that have already been approved by the FDA, against which biosimilar products are compared. There is 1 for Skytrofa.
Skytrofa (lonapegsomatropin-tcgd) - Ascendis Pharma Endocrinology Division A/S
| Formulation type | Strength |
|---|---|
| Single-Dose Cartridge | 0.7 mg |
| Single-Dose Cartridge | 1.4 mg |
| Single-Dose Cartridge | 1.8 mg |
| Single-Dose Cartridge | 11 mg |
| Single-Dose Cartridge | 13.3 mg |
| Single-Dose Cartridge | 2.1 mg |
| Single-Dose Cartridge | 2.5 mg |
| Single-Dose Cartridge | 3.6 mg |
| Single-Dose Cartridge | 3 mg |
| Single-Dose Cartridge | 4.3 mg |
| Single-Dose Cartridge | 5.2 mg |
| Single-Dose Cartridge | 6.3 mg |
| Single-Dose Cartridge | 7.6 mg |
| Single-Dose Cartridge | 9.1 mg |
References
- Thornton PS, Maniatis AK, Aghajanova E, et al. Weekly Lonapegsomatropin in Treatment-Naïve Children With Growth Hormone Deficiency: The Phase 3 heiGHt Trial. J Clin Endocrinol Metab. 2021 Oct 21;106(11):3184-3195. doi: 10.1210/clinem/dgab529. PMID: 34272849; PMCID: PMC8530727. Copy Download .nbib Format:
- Skytrofa Patient Brochure
- Skytrofa Package Insert
More about Skytrofa (lonapegsomatropin)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: growth hormones
- Breastfeeding
- En español
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.