Vizamyl: Package Insert / Prescribing Info
Package insert / product label
Generic name: flutemetamol f-18
Dosage form: injection
Drug class: Diagnostic radiopharmaceuticals
Medically reviewed by Drugs.com. Last updated on Sep 16, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
VIZAMYL™ (flutemetamol F 18 injection), for intravenous use
Initial U.S. Approval: 2013
Recent Major Changes
Indications and Usage for Vizamyl
VIZAMYL is a radioactive diagnostic drug indicated for positron emission tomography (PET) of the brain to estimate amyloid beta neuritic plaque density in adults with cognitive impairment for:
- Evaluation of Alzheimer's disease (AD) and other causes of cognitive decline
- Selection of patients who are indicated for amyloid beta-directed therapy as described in the prescribing information of the therapeutic products (1)
Vizamyl Dosage and Administration
- The recommended amount of radioactivity is 185 MBq (5 mCi) administered as a single intravenous bolus within 40 seconds in a total volume of up to 10 mL. (2.2)
- Follow injection with an intravenous flush of 5 mL to 15 mL of 0.9% sodium chloride injection. (2.2)
- Obtain 10-minute to 20-minute PET images starting approximately 60 minutes to 120 minutes after drug administration. (2.3)
- See full prescribing information for image interpretation and radiation dosimetry. (2.4, 2.5, 2.6)
Dosage Forms and Strengths
Injection: 150 MBq/mL (4.05 mCi/mL) of flutemetamol F 18 in up to 30 mL volume at reference date and time in a multiple-dose vial (3)
Contraindications
Known hypersensitivity to VIZAMYL or polysorbate 80 (4)
Warnings and Precautions
- Anaphylaxis and Other Serious Hypersensitivity Reactions: Always have emergency resuscitation equipment and trained personnel available at the time of VIZAMYL administration. (5.1)
- Risk of Image Misinterpretation and Other Errors: Image interpretation errors have been observed. (5.2)
- Radiation Risk: VIZAMYL contributes to a patient's long-term cumulative radiation exposure. Ensure safe drug handling to protect patients and health care providers from unintentional radiation exposure. Advise patients to hydrate before and after administration and to void frequently after administration. (2.1, 2.2, 5.3)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥ 1%) were flushing, increased blood pressure, headache, nausea, and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GE HealthCare at 1-800-654-0118 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Lactation: Temporarily discontinue breastfeeding. A lactating woman should pump and discard breast milk for 24 hours after VIZAMYL administration. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2025
Full Prescribing Information
1. Indications and Usage for Vizamyl
VIZAMYL is indicated for positron emission tomography (PET) of the brain to estimate amyloid beta neuritic plaque density in adults with cognitive impairment for:
- Evaluation of Alzheimer's disease (AD) and other causes of cognitive decline
- Selection of patients who are indicated for amyloid beta-directed therapy as described in the prescribing information of the therapeutic products
2. Vizamyl Dosage and Administration
2.1 Radiation Safety - Drug Handling
Handle VIZAMYL with appropriate safety measures to minimize radiation exposure during administration [see Warnings and Precautions (5.3)]. Use waterproof gloves and effective radiation shielding, including lead-glass syringe shields when handling and administering VIZAMYL.
Radiopharmaceuticals, including VIZAMYL, should be used by or under the control of healthcare providers who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
2.2 Recommended Dosage and Administration Instructions
Recommended Dosage
The recommended amount of activity of VIZAMYL is 185 MBq (5 mCi) in a total volume of up to 10 mL, administered as a single intravenous bolus within 40 seconds. The maximum mass dose is 20 mcg. Follow the injection with an intravenous flush of 5 mL to 15 mL of 0.9% sodium chloride injection.
Patient Preparation
Instruct patients to hydrate before and after VIZAMYL administration and to void frequently following VIZAMYL administration to reduce radiation exposure [see Warnings and Precautions (5.3)].
Administration
- Use aseptic technique and radiation shielding to withdraw and administer VIZAMYL.
- Visually inspect VIZAMYL for particulate matter and discoloration prior to administration. Do not use VIZAMYL if it contains particulate matter or if it is discolored.
- Do not dilute VIZAMYL.
- Calculate the necessary volume to administer based on calibration time and required dose.
- Measure the activity of VIZAMYL with a dose calibrator immediately prior to administration to the patient.
- Dispose of unused product in a safe manner in compliance with applicable regulations.
2.3 Image Acquisition Instructions
- Position the patient supine with the head positioned to center the brain, including the cerebellum, within a single field of view. The patient's head should be tilted so that the anterior commissure-posterior commissure (AC-PC) plane is at right angles to the bore-axis of the PET scanner, with the head positioned in a suitable head support. Tape or other flexible head restraints may be employed to reduce head movement.
- Acquire 10-minute to 20-minute PET images starting 60 minutes to 120 minutes after VIZAMYL administration using a PET scanner in 3-D mode with appropriate data corrections.
- Iterative or filtered back-projection reconstruction is recommended with a slice thickness of 2 mm to 4 mm, and matrix size of 128 × 128 with pixel sizes of approximately 2 mm. Where a post-smoothing filter is applied, full width at half maximum (FWHM) of not more than 5 mm is recommended; filter FWHM should be chosen to optimize the signal-to-noise ratio while preserving the sharpness of the reconstructed image.
2.4 Image Orientation and Display
Image Orientation
Orient axial and coronal images to show symmetry of brain structures, with equal heights of structures bilaterally. Orient sagittal images so that the head and neck are neither flexed nor extended; the anterior and posterior aspects of the corpus callosum should be parallel to the AC-PC line as shown in Figure 2.
Image Display
- Display images with all planes (axial, sagittal, and coronal planes) linked by crosshairs.
- Select a color scale that provides a progression of low through high intensity (e.g., rainbow or Sokoloff). The selected color scale should: (1) provide colors that allow the reader to discriminate signal intensity above and below the signal intensity of the pons; (2) provide a color for regions with little or no amyloid binding such as the cerebellar cortex; and (3) provide a range of at least five distinct colors above 50% to 60% of the peak signal intensity.
- Display the reference scale. Adjust the color scale to set the pons to approximately 90% maximum signal intensity. The cerebellar cortex should represent approximately 20% to 30% of peak signal intensity on both negative and positive VIZAMYL scans.
- Display axial brain slices sequentially from the bottom of the brain to the top and look for signs of atrophy.
- Systematically review signal intensity in the following brain regions (recommended plane) for image interpretation [see Dosage and Administration (2.5)]:
- Frontal lobes (axial, with optional sagittal plane view)
- Posterior cingulate and precuneus (sagittal, with optional coronal plane view)
- Lateral temporal lobes (axial, with optional coronal plane view)
- Inferolateral parietal lobes (coronal, with optional axial plane view)
- Striatum (axial, with optional sagittal plane view)
2.5 Image Interpretation
Visual Assessment
VIZAMYL images should be interpreted only by readers who successfully complete the training program provided by the manufacturer [see Warnings and Precautions (5.2)].
Perform image interpretation independently of the patient's clinical features, relying on the recognition of unique image features.
Interpret VIZAMYL images based upon the distribution of signal intensity within the cerebral cortex by comparing the signal intensity in the cortical gray matter and the adjacent white matter, or based on the signal intensity in the five regions mentioned above [see Dosage and Administration (2.4)]. The signal intensity in the cerebellum does not contribute to scan interpretation. For example, a positive scan may show retained cerebellar gray-white contrast even when the cortical gray-white contrast is lost. Among patients with clinically important amyloid beta neuritic plaques in the brain, the temporal lobes, parietal lobes, and striatum may not be as affected compared to other brain regions. Therefore, in some images, the signal in these regions may not be as intense as in the frontal lobes or the posterior cingulate and precuneus regions.
Some scans may be difficult to interpret due to image noise, suboptimal patient positioning, or over-smoothing of the reconstructed image. Atrophy may affect the interpretability of scans, particularly in the frontal, temporal, and parietal lobes. Other factors that may affect the ability to interpret VIZAMYL images include patient factors such as brain pathology, surgical changes, post-radiation therapy changes, and implants. For cases in which atrophy is apparent or suspected and there is uncertainty as to the location of the gray matter on the PET scan, examine the striatum for VIZAMYL signal as it is less affected by atrophy than other regions of the brain. If the patient's MRI or CT brain images are available, examine the CT or MRI images to clarify the relationship between VIZAMYL signal and gray matter anatomy [see Warnings and Precautions (5.2)].
Negative VIZAMYL Scan
Negative scans show more signal in white matter than in gray matter, creating clear gray-white matter contrast.
Specifically, a negative scan would have the following characteristics:
- frontal, lateral temporal, and inferolateral parietal lobes: gradual gradient from bright intensity of the white matter to lower intensity at the periphery of the brain; distinct sulci with concave surfaces (white matter sulcal pattern),
-
and -
posterior cingulate and precuneus: gray matter uptake below 50% to 60% of peak intensity; gap of lower intensity separates two hemispheres on coronal view, -
and -
striatum: approximately 50% of peak intensity or lower in the region between the higher intensities of the thalamus and frontal white matter (striatal "gap").
A negative scan indicates sparse to no neuritic plaques. In patients being evaluated for AD and other causes of cognitive decline who have not been treated with amyloid beta-directed therapy, a negative scan is inconsistent with a neuropathological diagnosis of AD at the time of image acquisition and reduces the likelihood that a patient's cognitive impairment is due to AD. A negative scan result does not preclude the accumulation of amyloid beta in the brain in the future.
Positive VIZAMYL Scan
Positive scans show at least one cortical region with reduction or loss of the normally distinct gray-white matter contrast. These scans have one or more regions with increased cortical gray matter signal (above 50% to 60% peak intensity) and/or reduced (or absent) gray-white matter contrast (white matter sulcal pattern is less distinct). A positive scan may have one or more regions in which gray matter signal is as intense or exceeds the intensity in adjacent white matter.
Specifically, a positive scan would have the following characteristics:
- frontal, lateral temporal, or inferolateral parietal lobes: high intensity seen to the periphery of the brain, with sharp reduction of intensity at the brain margin; sulci may not be distinct due to fill-in by high intensity gray matter resulting in a convex appearance of the surface at the edge of the brain,
-
or -
posterior cingulate and precuneus: gray matter uptake above 50% to 60% of peak intensity; high gray matter intensity that closes the gap between the two hemispheres on coronal view, -
or -
striatum: intensity above 50% to 60% of peak intensity; gap between thalamus and frontal white matter not distinct.
If any one of the brain regions systematically reviewed for signal intensity is positive in either hemisphere, then the scan is considered positive [see Dosage and Administration (2.4)]. Otherwise, the scan is considered negative.
A positive scan establishes the presence of moderate to frequent neuritic plaques. Neuropathological examination has shown that moderate to frequent amyloid beta neuritic plaques are present in patients with AD, but may also be present in patients with other types of neurologic conditions as well as older people with normal cognition.
Figures 1, 2, and 3 provide examples of negative and positive scans.
Figure 1: Axial View of Negative (left) and Positive (right) VIZAMYL Scans. The axial slices that cut through the frontal pole and inferior aspect of the splenium are shown using a rainbow color scale. The left image shows a white matter sulcal pattern at the frontal (f) and lateral temporal (lt) regions with a color intensity that tapers to the periphery, as well as less signal intensity in the striatal region(s). The right image shows absence of the white matter sulcal pattern with intensity radiating to a sharply defined convex edge, as well as more signal intensity in the striatum. In both the frontal and lateral temporal regions, the intensity is higher in the gray matter regions of the right image compared to those of the left image.
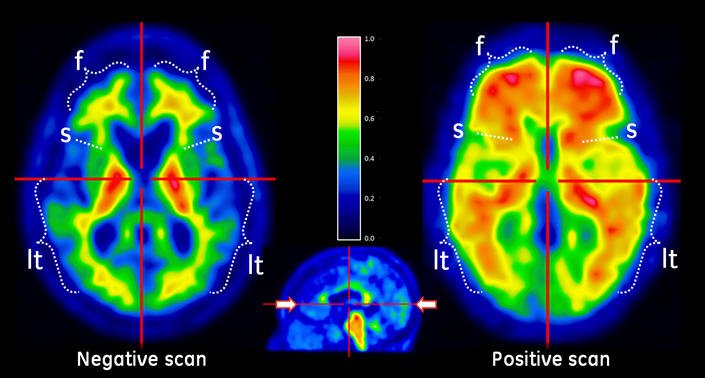
Figure 2: Sagittal View of Negative (left) and Positive (right) VIZAMYL Scans. The sagittal slices are slightly off midline in one hemisphere and shown using a rainbow color scale. In the posterior cingulate (pc) region, which is superior and posterior to the corpus callosum (cc), the left image shows signal intensity below 50% of peak intensity whereas the right image shows intensity above 60% of peak intensity. The pons (p) is set to approximately 90% of the maximum intensity.
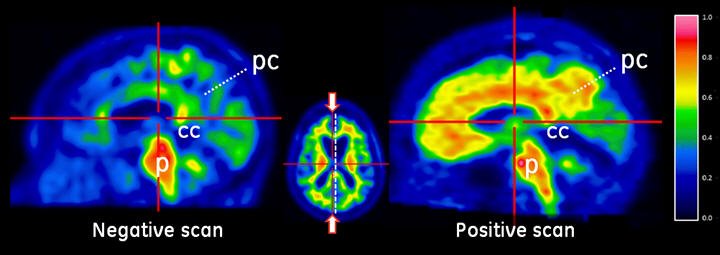
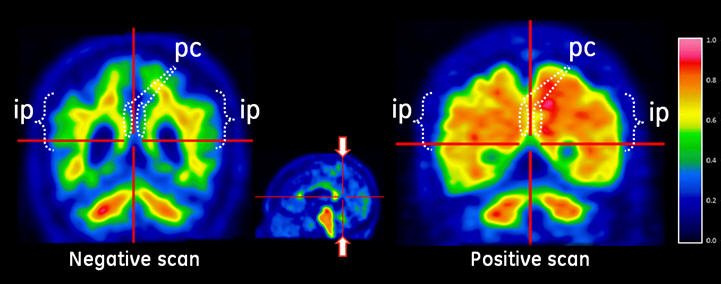
Figure 3: Coronal View of Negative (left) and Positive (right) VIZAMYL Scans. The coronal slices are located posterior to the corpus callosum. The left image shows a white matter sulcal pattern in the inferior parietal (ip) regions that is not evident in the right image. Relative to the left image, the right image shows increased signal intensity in the posterior cinguli (pc) and increased radial extent of high intensity to the lateral surfaces of the parietal lobes particularly evident in the inferior parietal regions.
Quantitative Analysis
Quantification of amyloid beta neuritic plaque levels (e.g., Centiloid scale or standardized uptake value ratio (SUVR)) can be used in conjunction with visual assessment and performed with FDA-authorized software indicated for quantification of brain amyloid PET scans. Refer to the drug manufacturer's training materials for qualitative and quantitative assessment and to the software manufacturers' documentation for software operation.
2.6 Radiation Dosimetry
Estimated radiation absorbed doses for adults following intravenous injection of VIZAMYL are shown in Table 1.
Values were calculated from human biodistribution data using OLINDA/EXM software and assuming emptying of the urinary bladder at 3.5-hour intervals.
The whole-body effective dose resulting from administration of 185 MBq (5 mCi) of VIZAMYL to an adult is estimated to be 5.9 mSv. When PET/CT is performed, exposure to radiation will increase by an amount dependent on the settings used in the CT acquisition.
| Organ/Tissue | Absorbed Dose Per Unit Administered Activity (microGy/MBq) |
|---|---|
| Adrenals | 13 |
| Brain | 11 |
| Breasts | 5 |
| Gallbladder wall | 287 |
| Heart wall | 14 |
| Kidneys | 31 |
| Liver | 57 |
| Lower large intestine wall | 42 |
| Lungs | 16 |
| Muscle | 9 |
| Osteogenic cells | 11 |
| Ovaries | 25 |
| Pancreas | 15 |
| Red marrow | 13 |
| Skin | 5 |
| Small intestine wall | 102 |
| Spleen | 15 |
| Stomach wall | 12 |
| Testes | 8 |
| Thymus | 6 |
| Thyroid | 6 |
| Upper large intestine wall | 117 |
| Urinary bladder wall | 145 |
| Uterus | 25 |
| Total body | 12 |
| Effective Dose | 32 (microSv/MBq) |
3. Dosage Forms and Strengths
Injection: 150 MBq/mL (4.05 mCi/mL) of flutemetamol F 18 in up to 30 mL volume at reference date and time as a clear, colorless to slightly yellow solution in a multiple-dose vial.
4. Contraindications
VIZAMYL is contraindicated in patients with a history of hypersensitivity reaction to VIZAMYL or polysorbate 80 [see Warnings and Precautions (5.1)].
5. Warnings and Precautions
5.1 Anaphylaxis and Other Serious Hypersensitivity Reactions
Serious hypersensitivity reactions including anaphylaxis, presenting with flushing, dyspnea, and hypotension, have been observed within minutes following VIZAMYL administration. These reactions may occur in patients with no history of exposure to VIZAMYL [see Adverse Reactions (6.1, 6.2)].
Obtain a history of allergy or hypersensitivity reactions. Always have resuscitation equipment and trained personnel immediately available at the time of VIZAMYL administration. If a hypersensitivity reaction is suspected, immediately discontinue the injection and initiate appropriate therapy. VIZAMYL is contraindicated in patients with a history of hypersensitivity to VIZAMYL or polysorbate 80 [see Contraindications (4)].
5.2 Risk of Image Misinterpretation and Other Errors
Errors may occur in the estimation of amyloid beta neuritic plaque density during VIZAMYL image interpretation [see Clinical Studies (14)].
The use of clinical information in the interpretation of VIZAMYL images has not been evaluated and may lead to an inaccurate assessment. Extensive brain atrophy and motion artifacts that distort the image may limit the ability to distinguish gray and white matter on a VIZAMYL scan.
Perform image interpretation independently of the patient's clinical information. For cases where there is uncertainty as to the location of cortical signal, use co-registered anatomical imaging to improve localization of signal or examine the striatum for VIZAMYL signal as it is less affected by atrophy [see Dosage and Administration (2.5)].
5.3 Radiation Risk
VIZAMYL contributes to a patient's overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Ensure safe drug handling to protect patients and health care providers from unintentional radiation exposure. Advise patients to hydrate before and after administration and to void frequently after administration [see Dosage and Administration (2.1, 2.2)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reaction is described elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of VIZAMYL was evaluated in 761 adult subjects who received VIZAMYL by intravenous injection in clinical trials. Most subjects (70%) received a dose of 185 MBq (5 mCi). The subjects had a mean age of 62 years (range 18 years to 93 years); 45% of the subjects were male and 91% were White.
A serious hypersensitivity reaction characterized by flushing, dyspnea, and chest pressure was reported within minutes following VIZAMYL administration in one subject who recovered with treatment.
Adverse reactions reported in ≥ 1% of subjects from the clinical trials are shown in Table 2.
| Adverse Reaction | VIZAMYL N=761 % |
|---|---|
| Flushing | 2 |
| Increased blood pressure | 2 |
| Headache | 1 |
| Nausea | 1 |
| Dizziness | 1 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of VIZAMYL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available data on VIZAMYL use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with flutemetamol F 18 to evaluate its effect on female reproduction and embryo-fetal development.
All radiopharmaceuticals, including VIZAMYL, have the potential to cause fetal harm depending on the stage of fetal development and the magnitude of the radiation dose. If considering VIZAMYL administration to a pregnant woman, inform the patient about the potential for adverse pregnancy outcomes based on the radiation dose from the drug and the gestational timing of exposure.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of flutemetamol F 18 or metabolites in human milk or its effects on the breastfed infant or milk production. Exposure of VIZAMYL to a breastfed infant can be minimized by temporary discontinuation of breastfeeding (see Clinical Considerations). The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for VIZAMYL and any potential adverse effects on the breastfed child from VIZAMYL or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of VIZAMYL have not been established in pediatric patients.
8.5 Geriatric Use
Of the 761 subjects in clinical studies of VIZAMYL, 447 (59%) subjects were 65 years of age and older, while 246 (32%) subjects were 75 years of age and older. No overall differences in safety or effectiveness were observed between subjects 65 years of age and older and younger adult subjects.
10. Overdosage
The major risks of overdose relate predominantly to increased radiation exposure, with long-term risks for neoplasia. In the event of administration of a radiation overdose with VIZAMYL, hydration and frequent urination should be encouraged to minimize radiation exposure to the subject. It is unknown whether or not flutemetamol is dialyzable.
11. Vizamyl Description
11.1 Drug Characteristics
VIZAMYL (flutemetamol F 18 injection) is a radioactive diagnostic drug for intravenous use.
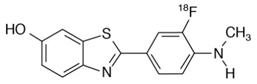
Chemically, flutemetamol F 18 is 2-[3-[18F]fluoro-4-(methylamino) phenyl]-6-benzothiazolol. It has the molecular formula C14H1118FN2OS, the molecular weight 273.32, and the following structural formula:
VIZAMYL is a sterile, non-pyrogenic, clear, colorless to slightly yellow solution. Each mL contains up to 2 mcg of flutemetamol and 150 MBq (4.05 mCi) of flutemetamol F 18 at reference date and time with the following inactive ingredients: 70 microL ethanol, 9 mg sodium chloride, and 4.98 mg polysorbate 80 (w/v) in 0.014 M aqueous phosphate buffer. The pH of the solution is between 6 and 8.5.
11.2 Nuclear Physical Characteristics
Fluorine-18 (F 18) decays by positron emission (ß+ decay, 96.7%) and orbital electron capture (3.3%) to stable oxygen-18 and has a physical half-life of 109.8 minutes. The principal photons useful for diagnostic imaging are the coincident pair of 511 keV gamma photons, resulting from the interaction of the emitted positron with an electron (Table 3).
| Radiation | Energy Level (keV) | Abundance (%) |
|---|---|---|
| Positron | 249.8 | 96.7 |
| Gamma | 511 | 193.4 |
The point source air-kerma rate constant for F 18 is 3.74E -17 Gy m2/(Bq s); this coefficient was formerly defined as the specific gamma-ray constant of 5.7 R/hr/mCi at 1 cm. The first half-value thickness of lead (Pb) for F 18 gamma rays is approximately 6 mm. The relative reduction of radiation emitted by F 18 that results from various thicknesses of lead shielding is shown in Table 4. The use of ~8 cm of Pb will decrease the radiation transmission (i.e., exposure) by a factor of about 10,000.
| Shield Thickness cm of Lead (Pb) | Coefficient of Attenuation |
|---|---|
| 0.6 | 0.5 |
| 2 | 0.1 |
| 4 | 0.01 |
| 6 | 0.001 |
| 8 | 0.0001 |
For use in correcting for physical decay of this radionuclide, the fractions remaining at selected intervals after calibration are shown in Table 5.
| Minutes | Fraction Remaining |
|---|---|
| 0 | 1.00 |
| 15 | 0.909 |
| 30 | 0.826 |
| 60 | 0.683 |
| 110 | 0.500 |
| 220 | 0.250 |
| 440 | 0.060 |
12. Vizamyl - Clinical Pharmacology
12.1 Mechanism of Action
Flutemetamol F 18 binds to amyloid beta plaques in the brain and the F 18 isotope produces a positron signal that is detected by a PET scanner. In in vitro binding studies using postmortem human brain homogenates containing fibrillar amyloid beta, the dissociation constant (Kd) for flutemetamol was 6.7 nM.
Selectivity of 3H-flutemetamol binding in postmortem human brain sections was demonstrated using autoradiography, silver-stained protein, and immunohistochemistry (monoclonal antibody to amyloid beta) correlation studies.
12.2 Pharmacodynamics
Following intravenous injection, flutemetamol F 18 diffuses across the human blood-brain barrier and produces a radioactivity signal detectable throughout the brain. Subsequently, cerebral perfusion decreases the brain flutemetamol F 18 content, with differential retention of the drug in cortical areas that contain amyloid beta aggregates compared to areas that lack the aggregates. The time-activity curves for flutemetamol F 18 in the brain of subjects with positive scans shows continual signal increases from time zero through 30 minutes post administration, with stable values thereafter up to at least 120 minutes post-injection. Differences in signal intensity between brain regions showing specific and non-specific flutemetamol F 18 uptake form the basis for the image interpretation method [see Dosage and Administration (2.5)].
The test-retest distribution of flutemetamol F 18 was evaluated in five subjects with probable AD who underwent two administrations of flutemetamol F 18 (followed by PET scans) separated by a time period of 1 to 4 weeks. Images were shown to maintain signal distribution reproducibility when evaluated semi-quantitatively using an automated assessment of SUVR in pre-specified cortical regions.
12.3 Pharmacokinetics
Following intravenous administration of 185 MBq (5 mCi) of VIZAMYL in healthy subjects, flutemetamol F 18 plasma concentrations declined by approximately 75% in the first 20 minutes post-injection, and by approximately 90% in the first 180 minutes. The F 18 in circulation during the 30-minute to 120-minute imaging window was principally in the form of flutemetamol metabolites. Excretion was approximately 37% renal (28% to 45%; n=6) and 52% hepatobiliary (40% to 65%; n=6).
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not been performed to evaluate the carcinogenicity or reproductive toxicity potential of flutemetamol F 18.
Mutagenesis
Flutemetamol was positive for mutagenicity in two in vitro assays: the bacterial reverse mutation assay (Ames test) and the mouse lymphoma assay.
Flutemetamol was negative for genotoxicity after in vivo exposure in rats to flutemetamol at the highest cumulative dose level tested, as measured in bone marrow micronucleus assays (157 and 27 mcg/kg/day for 2 days and 14 days, respectively) and an unscheduled DNA synthesis assay in rat hepatocytes (39 mcg/kg/day).
14. Clinical Studies
14.1 Evaluation of AD and Other Causes of Cognitive Decline
The effectiveness of VIZAMYL was evaluated in two single-arm clinical studies (i.e., Studies 1 and 2) in adult subjects with a range of cognitive function, including some terminally ill subjects who had agreed to participate in a postmortem brain donation program as well as healthy subjects. Subjects underwent a VIZAMYL injection and scan. The images were interpreted using a clinically applicable binary image interpretation method (negative or positive) by five independent readers blinded to all clinical information [see Dosage and Administration (2.3, 2.4, 2.5)]. PET images were reviewed first without, and subsequently with, brain CT or MRI images. Before image interpretation, all readers underwent in-person training or electronic media training.
To determine the agreement between the in vivo VIZAMYL image results and the postmortem amyloid beta neuritic plaque density, VIZAMYL results (negative or positive) were pre-specified to correspond with specific histopathology-derived plaque density scores, based upon a modification of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) criteria (Table 6), which use neuritic plaque counts as a necessary pathological feature of AD. Plaques were counted on microscope slides with modified Bielschowsky silver stained tissue sections. The global neuritic plaque density score for each subject was determined by averaging across the scores (0-3) for five gray matter fields per slide and then across six slides for each of eight regions; if any one region had a score of greater than 1.5, the subject was classified as positive for amyloid beta.
| Histopathology Categorization | VIZAMYL PET Read | |
|---|---|---|
| Neuritic Plaque Counts | CERAD Score | |
| 0 | None (0) | Negative |
| 1 to 5 | Sparse (1) | |
| 6 to 19 | Moderate (2) | Positive |
| >20 | Frequent (3) | |
Study 1 evaluated performance characteristics (sensitivity and specificity) in terminally ill subjects by comparing the premortem VIZAMYL PET images to a postmortem truth standard of cortical amyloid beta neuritic plaque density. A total of 180 subjects were dosed with VIZAMYL and 176 were imaged. The mean age was 80 years (range 47 to 98 years) and 57% of the subjects were female. By medical history, 135 subjects had dementia, one subject had memory loss of unspecified nature, and 44 subjects had no cognitive impairment. Among the imaged subjects, 68 subjects who died during the study and had cerebral cortical amyloid status determined were included in the primary analysis. The time interval between the VIZAMYL scan and death ranged from 0 to 13 months, with a median of 2.6 months, and was less than one year for 66 subjects and between 12 to 13 months for 2 subjects. At autopsy, the global brain neuritic plaque density category (CERAD score as in Table 6) was available for 67/68 subjects: frequent (n = 19); moderate (n = 22); sparse (n = 14); and none (n = 12).
Study 2 evaluated inter-reader and intra-reader reproducibility of image interpretation using images from subjects with a truth standard (68 subjects who underwent an autopsy in Study 1 and 36 subjects who had known or suspected normal pressure hydrocephalus with in vivo brain biopsy) and subjects without a truth standard (80 subjects with amnestic mild cognitive impairment (aMCI), 33 subjects with probable AD (pAD), 28 cognitively normal subjects 55 years of age and older, and 31 young healthy subjects). Additionally, intra-reader reproducibility was assessed with images from 29 subjects (10%). Among the 276 subjects, the mean age was 68 years (range 20 to 95 years), 136 were females, and 251 were White. Readers were naïve to all forms of amyloid PET imaging and underwent electronic media training.
Among subjects who underwent autopsy (n=68; 43 positive and 25 negative based on histopathology), the sensitivity using the majority interpretation of the readers trained using electronic media was 93% (95% CI: 81%, 99%) and specificity was 84% (95% CI: 64%, 96%). The median (and range) of correct, false negative, and false positive reads were 59 (51, 61), 5 (3, 8), 3 (2, 14), respectively, for in-person training; and were 60 (55 to 61), 3 (3 to 6), 4 (2 to 10), respectively, for electronic media training.
Table 7 shows inter-reader reproducibility results among readers for various subject groups in Study 2. Inter-reader reproducibility analyses showed an overall Fleiss' kappa statistic of 0.83 (95% CI: 0.79, 0.86), which met the pre-specified success criterion (95% CI lower bound > 0.60). Intra-reader reproducibility analyses showed that between the two readings for each of the 29 duplicate scans, one of the five readers had complete agreement for all 29 scans, two readers had discordant reads for a single scan, and two readers had discordant reads for two scans. Intra-reader reproducibility for a subgroup of eight scans from aMCI subjects showed that all five readers had complete agreement for all duplicate scans.
| Subject Group by Cognitive Status and Truth Standard (TS) | Positive Scans, N† | Kappa (95% CI) | Percent of Scans with Inter-Reader Agreement | ||
|---|---|---|---|---|---|
| 3 of 5 readers agreed | 4 of 5 readers agreed | 5 of 5 readers agreed | |||
| pAD: probable AD; aMCI: amnestic MCI | |||||
|
|||||
| All subjects, n=276 | 139 | 0.83 (0.79, 0.86) | 5 | 14 | 81 |
| All subjects with TS, n=104 (68 autopsy; 36 biopsy) | 58 | 0.74 (0.68, 0.80) | 6 | 24 | 70 |
| All subjects without TS, n=172 | 76 | 0.88 (0.83, 0.92) | 5 | 8 | 87 |
| pAD, n=63 (30 autopsy; 33 no TS) | 47 | 0.88 (0.80, 0.96) | 3 | 6 | 90 |
| aMCI without TS, n=80 | 45 | 0.89 (0.82, 0.96) | 4 | 7 | 89 |
| Other non-AD dementia with TS, n=53 (17 autopsy; 36 biopsy‡) | 27 | 0.71 (0.63, 0.80) | 8 | 25 | 68 |
| Cognitively normal with TS, n=21 (21 autopsy) | 10 | 0.64 (0.5, 0.77) | 5 | 38 | 57 |
| Cognitively normal 55 years of age and older without TS, n=28 | 2 | 0.46 (0.34, 0.57) | 4 | 14 | 82 |
14.2 Selection of Patients Indicated for Amyloid Beta-Directed Therapy
Refer to the prescribing information of the amyloid beta-directed therapy for description of clinical trials in which the efficacy of amyloid beta PET for selecting patients has been established.
Brain amyloid beta PET scans have been used to assess reduction of plaque in some clinical trials of amyloid beta-directed therapies as also described in the prescribing information of the therapeutic products.
16. How is Vizamyl supplied
How Supplied
VIZAMYL (flutemetamol F 18 injection) is a clear, colorless to slightly yellow solution supplied at a concentration of 150 MBq/mL (4.05 mCi/mL) of flutemetamol F 18 in up to 30 mL volume at reference date and time in a shielded multiple-dose glass vial (NDC 17156-067-30).
Storage and Handling
Store VIZAMYL in the original container within radiation shielding at 2°C to 30°C (36°F to 86°F).
VIZAMYL does not contain a preservative.
Do not use after the expiry date and time stated on the label. VIZAMYL multiple-dose vial expires 10 hours after end of synthesis (EOS).
Dispose of any unused product in accordance with all federal, state, and local laws and institutional requirements.
This preparation is for use by persons licensed by the Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State.
17. Patient Counseling Information
Anaphylaxis and Other Serious Hypersensitivity Reactions
Inform patients of the risk of hypersensitivity reactions, including anaphylaxis, and instruct them to alert healthcare providers immediately if they experience signs and symptoms of a hypersensitivity reaction [see Warnings and Precautions (5.1)].
Radiation Risk
Advise patients of the radiation risk of VIZAMYL. Instruct patients to increase their level of hydration before and after receiving VIZAMYL and to void frequently following administration [see Warnings and Precautions (5.3)].
Pregnancy
Inform pregnant women of the potential risks of fetal exposure to radiation doses with VIZAMYL [see Use in Specific Populations (8.1)].
Lactation
Advise a lactating woman to temporarily discontinue breastfeeding and to pump and discard breast milk for 24 hours after VIZAMYL administration to minimize radiation exposure to the breastfed infant [see Use in Specific Populations (8.2)].
Manufactured for
GE Healthcare, Medi-Physics, Inc.
Arlington Heights, IL 60004
U.S.A.
VIZAMYL is a trademark of GE HealthCare or one of its subsidiaries.
GE is a trademark of General Electric Company used under trademark license.
© 2025 GE HealthCare
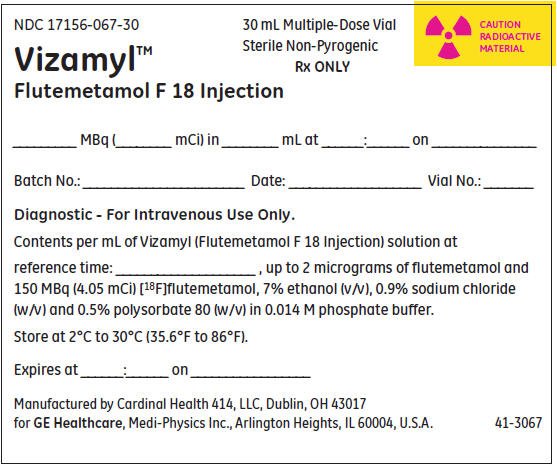
PRINCIPAL DISPLAY PANEL - 30 mL Vial Label
NDC 17156-067-30
Multiple-Dose Vial
Sterile, Non-Pyrogenic
Rx ONLY
CAUTION
RADIOACTIVE
MATERIAL
Vizamyl™
(flutemetamol F 18 injection)
150 MBq/mL (4.05 mCi/mL) at reference date and time
Diagnostic - For Intravenous Use Only.
Batch No.: ___________________ Date: ______________________Vial No.:_______________
Each mL contains up to 2 micrograms of flutemetamol and 150 MBq (4.05 mCi) flutemetamol F
18 at reference date and time, 70 microL ethanol, 9 mg sodium chloride, and 4.98 mg
polysorbate 80 (w/v) in 0.014 M aqueous phosphate buffer.
Store at 2°C to 30°C (36°F to 86°F). Recommended dosage: See prescribing information.
Distributed by GE HealthCare, Medi-Physics Inc., Arlington Heights, IL 60004, U.S.A.
40-3067A
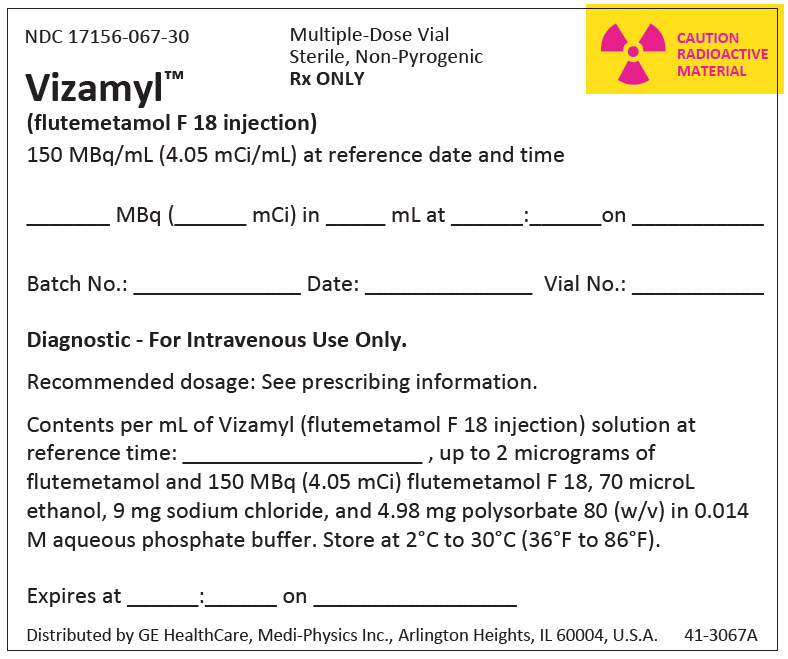
PRINCIPAL DISPLAY PANEL - 30 mL Vial Container Label
NDC 17156-067-30
Multiple-Dose Vial
Sterile, Non-Pyrogenic
Rx ONLY
CAUTION
RADIOACTIVE
MATERIAL
Vizamyl™
(flutemetamol F 18 injection)
150 MBq/mL (4.05 mCi/mL) at reference date and time
_______ MBq (________ mCi) in ________ mL at ______:______on _______________
Batch No.: _______________________ Date: ___________________ Vial No.: _______
Diagnostic - For Intravenous Use Only.
Recommended dosage: See prescribing information.
Contents per mL of Vizamyl (flutemetamol F 18 injection) solution at
reference time: ____________________ , up to 2 micrograms of
flutemetamol and 150 MBq (4.05 mCi) flutemetamol F 18, 70 microL
ethanol, 9 mg sodium chloride, and 4.98 mg polysorbate 80 (w/v) in 0.014
M aqueous phosphate buffer. Store at 2°C to 30°C (36°F to 86°F).
Expires at ______:______ on _________________
Distributed by GE HealthCare, Medi-Physics Inc., Arlington Heights, IL 60004, U.S.A.
41-3067A
| VIZAMYL
flutemetamol f-18 solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Medi-Physics, Inc. dba GE Healthcare (095263729) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 414, LLC (Sacramento, CA) | 165486861 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 414, LLC (Phoenix, AZ) | 833114734 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Precision Nuclear, LLC (Johnson City, TN) | 879283633 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health, Inc. (East Lansing, MI) | 963972166 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 414, LLC (Colton, CA) | 964767656 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 414, LLC (Beltsville, MD) | 964767771 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 414, LLC (Seattle, WA) | 964768126 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 414, LLC (Dallas, TX) | 964768282 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 414, LLC (Tampa, FL) | 964768340 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 414, LLC (Charlotte, NC) | 964768373 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 418, Inc. (Aurora, CO) | 149029253 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health, Inc. (Houston, TX) | 826800364 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health, Inc. (Glendale Heights, IL) | 033231923 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 414, LLC (New Orleans, LA) | 080130462 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 414, LLC (E. Hartford, CT) | 964768233 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 414, LLC (Ft. Lauderdale, FL) | 964767722 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health, Inc. (Las Vegas, NV) | 039587455 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| The University of Utah DBA Cyclotron Radiochemistry Lab Huntsman Cancer Institute | 018432646 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Essential Isotopes, LLC. (Colombia, MO) | 010753961 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmalogic Colorado, LLC | 117608150 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GE Healthcare AS (Oslo, Norway) | 515048908 | MANUFACTURE(17156-067) , ANALYSIS(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GE Healthcare Inc. (Medi-Physics, Inc. dba GE Healthcare) | 095263729 | ANALYSIS(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health, Inc. (Rochester, NY ) | 964768415 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmalogic New York City, LLC | 118410433 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmalogic Cincinnati, OH | 118408248 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmalogic Los Angeles, LLC | 119024924 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 414, LLC (Columbus, OH ) | 103283375 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 414, LLC (Omaha, NE) | 964768456 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 414, LLC (Birmingham, AL) | 963512384 | POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) | |
More about Vizamyl (flutemetamol f-18)
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: diagnostic radiopharmaceuticals
- Breastfeeding
