Prostin VR Pediatric: Package Insert / Prescribing Info
Package insert / product label
Generic name: alprostadil
Dosage form: injection, solution
Drug classes: Impotence agents, Vasodilators
J Code (medical billing code): J0270 (Per 1.25 mcg, injection)
Medically reviewed by Drugs.com. Last updated on Sep 25, 2024.
On This Page
WARNING
Apnea is experienced by about 10 to 12% of neonates with congenital heart defects treated with PROSTIN VR PEDIATRIC Sterile Solution. Apnea is most often seen in neonates weighing less than 2 kg at birth and usually appears during the first hour of drug infusion. Therefore, respiratory status should be monitored throughout treatment, and PROSTIN VR PEDIATRIC should be used where ventilatory assistance is immediately available.
Prostin VR Pediatric Description
PROSTIN VR PEDIATRIC Sterile Solution for intravascular infusion contains 500 micrograms alprostadil, more commonly known as prostaglandin E1, in 1.0 mL dehydrated alcohol.
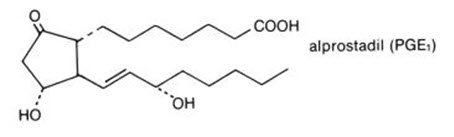
The chemical name for alprostadil is (11α,13E,15S)-11,15 dihydroxy-9-oxo-prost-13-en-1-oic acid, and the molecular weight is 354.49.
Alprostadil is a white to off-white crystalline powder with a melting point between 110° and 116°C. Its solubility at 35°C is 8000 micrograms per 100 mL double distilled water.
Structural Formula
Prostin VR Pediatric - Clinical Pharmacology
Alprostadil (prostaglandin E1) is one of a family of naturally occurring acidic lipids with various pharmacologic effects. Vasodilation, inhibition of platelet aggregation, and stimulation of intestinal and uterine smooth muscle are among the most notable of these effects. Intravenous doses of 1 to 10 micrograms of alprostadil per kilogram of body weight lower the blood pressure in mammals by decreasing peripheral resistance. Reflex increases in cardiac output and rate accompany the reduction in blood pressure.
Smooth muscle of the ductus arteriosus is especially sensitive to alprostadil, and strips of lamb ductus markedly relax in the presence of the drug. In addition, administration of alprostadil reopened the closing ductus of new-born rats, rabbits, and lambs. These observations led to the investigation of alprostadil in infants who had congenital defects which restricted the pulmonary or systemic blood flow and who depended on a patent ductus arteriosus for adequate blood oxygenation and lower body perfusion.
In infants with restricted pulmonary blood flow, about 50% responded to alprostadil infusion with at least a 10 torr increase in blood pO2 (mean increase about 14 torr and mean increase in oxygen saturation about 23%). In general, patients who responded best had low pretreatment blood pO2 and were 4 days old or less.
In infants with restricted systemic blood flow, alprostadil often increased pH in those having acidosis, increased systemic blood pressure, and decreased the ratio of pulmonary artery pressure to aortic pressure.
Alprostadil must be infused continuously because it is very rapidly metabolized. As much as 80% of the circulating alprostadil may be metabolized in one pass through the lungs, primarily by β- and ω- oxidation. The metabolites are excreted primarily by the kidney, and excretion is essentially complete within 24 hours after administration. No unchanged alprostadil has been found in the urine, and there is no evidence of tissue retention of alprostadil or its metabolites.
Indications and Usage for Prostin VR Pediatric
PROSTIN VR PEDIATRIC Sterile Solution is indicated for palliative, not definitive, therapy to temporarily maintain the patency of the ductus arteriosus until corrective or palliative surgery can be performed in neonates who have congenital heart defects and who depend upon the patent ductus for survival. Such congenital heart defects include pulmonary atresia, pulmonary stenosis, tricuspid atresia, tetralogy of Fallot, interruption of the aortic arch, coarctation of the aorta, or transposition of the great vessels with or without other defects.
In infants with restricted pulmonary blood flow, the increase in blood oxygenation is inversely proportional to pretreatment pO2 values; that is, patients with low pO2 values respond best, and patients with pO2 values of 40 torr or more usually have little response.
PROSTIN VR PEDIATRIC should be administered only by trained personnel in facilities that provide pediatric intensive care.
Warnings
See WARNING box.
NOTE: PROSTIN VR PEDIATRIC Sterile Solution must be diluted before it is administered. See dilution instructions in DOSAGE AND ADMINISTRATION section.
The administration of PROSTIN VR PEDIATRIC to neonates may result in gastric outlet obstruction secondary to antral hyperplasia. This effect appears to be related to duration of therapy and cumulative dose of the drug. Neonates receiving PROSTIN VR PEDIATRIC at recommended doses for more than 120 hours should be closely monitored for evidence of antral hyperplasia and gastric outlet obstruction.
PROSTIN VR PEDIATRIC should be infused for the shortest time and at the lowest dose that will produce the desired effects. The risks of long-term infusion of PROSTIN VR PEDIATRIC should be weighed against the possible benefits that critically ill infants may derive from its administration.
Precautions
General Precautions
Cortical proliferation of the long bones, first observed in dogs, has also been observed in infants during long-term infusions of alprostadil. The cortical proliferation in infants regressed after withdrawal of the drug.
In infants treated with PROSTIN VR PEDIATRIC at the usual doses for 10 hours to 12 days and who died of causes unrelated to ductus structural weakness, tissue sections of the ductus and pulmonary arteries have shown intimal lacerations, a decrease in medial muscularity and disruption of the medial and internal elastic lamina. Localized and aneurysmal dilatations and vessel wall edema also were seen compared to a series of pathological specimens from infants not treated with PROSTIN VR PEDIATRIC. The incidence of such structural alterations has not been defined.
Because alprostadil inhibits platelet aggregation, use PROSTIN VR PEDIATRIC cautiously in neonates with bleeding tendencies.
PROSTIN VR PEDIATRIC should not be used in neonates with respiratory distress syndrome. A differential diagnosis should be made between respiratory distress syndrome (hyaline membrane disease) and cyanotic heart disease (restricted pulmonary blood flow). If full diagnostic facilities are not immediately available, cyanosis (pO2 less than 40 torr) and restricted pulmonary blood flow apparent on an X-ray are appropriate indicators of congenital heart defects.
Necessary Monitoring
In all neonates, arterial pressure should be monitored intermittently by umbilical artery catheter, auscultation, or with a Doppler transducer. Should arterial pressure fall significantly, decrease the rate of infusion immediately.
In infants with restricted pulmonary blood flow, measure efficacy of PROSTIN VR PEDIATRIC by monitoring improvement in blood oxygenation. In infants with restricted systemic blood flow, measure efficacy by monitoring improvement of systemic blood pressure and blood pH.
Drug Interactions
No drug interactions have been reported between PROSTIN VR PEDIATRIC and the therapy standard in neonates with restricted pulmonary or systemic blood flow. Standard therapy includes antibiotics, such as penicillin and gentamicin; vasopressors, such as dopamine and isoproterenol; cardiac glycosides; and diuretics, such as furosemide.
Adverse Reactions/Side Effects
Central Nervous System
Apnea has been reported in about 12% of the neonates treated. (See WARNING box.) Other common adverse reactions reported have been fever in about 14% of the patients treated and seizures in about 4%. The following reactions have been reported in less than 1% of the patients: cerebral bleeding, hyperextension of the neck, hyperirritability, hypothermia, jitteriness, lethargy, and stiffness.
Cardiovascular System
The most common adverse reactions reported have been flushing in about 10% of patients (more common after intraarterial dosing), bradycardia in about 7%, hypotension in about 4%, tachycardia in about 3%, cardiac arrest in about 1%, and edema in about 1%. The following reactions have been reported in less than 1% of the patients: congestive heart failure, hyperemia, second degree heart block, shock, spasm of the right ventricle infundibulum, supraventricular tachycardia, and ventricular fibrillation.
Respiratory System
The following reactions have been reported in less than 1% of the patients: bradypnea, bronchial wheezing, hypercapnia, respiratory depression, respiratory distress, and tachypnea.
Gastrointestinal System
See WARNINGS
The most common adverse reaction reported has been diarrhea in about 2% of the patients. The following reactions have been reported in less than 1% of the patients: gastric regurgitation, and hyperbilirubinemia.
Related/similar drugs
Overdosage
Apnea, bradycardia, pyrexia, hypotension, and flushing may be signs of drug overdosage. If apnea or bradycardia occurs, discontinue the infusion, and provide appropriate medical treatment. Caution should be used in restarting the infusion. If pyrexia or hypotension occurs, reduce the infusion rate until these symptoms subside. Flushing is usually a result of incorrect intraarterial catheter placement, and the catheter should be repositioned.
Prostin VR Pediatric Dosage and Administration
The preferred route of administration for PROSTIN VR PEDIATRIC Sterile Solution is continuous intravenous infusion into a large vein. Alternatively, PROSTIN VR PEDIATRIC may be administered through an umbilical artery catheter placed at the ductal opening. Increases in blood pO2 (torr) have been the same in neonates who received the drug by either route of administration.
Begin infusion with 0.05 to 0.1 micrograms alprostadil per kilogram of body weight per minute. A starting dose of 0.1 micrograms per kilogram of body weight per minute is the recommended starting dose based on clinical studies; however, adequate clinical response has been reported using a starting dose of 0.05 micrograms per kilogram of body weight per minute. After a therapeutic response is achieved (increased pO2 in infants with restricted pulmonary blood flow or increased systemic blood pressure and blood pH in infants with restricted systemic blood flow), reduce the infusion rate to provide the lowest possible dosage that maintains the response. This may be accomplished by reducing the dosage from 0.1 to 0.05 to 0.025 to 0.01 micrograms per kilogram of body weight per minute. If response to 0.05 micrograms per kilogram of body weight per minute is inadequate, dosage can be increased up to 0.4 micrograms per kilogram of body weight per minute although, in general, higher infusion rates do not produce greater effects.
Dilution Instructions
To prepare infusion solutions, dilute 1 mL of PROSTIN VR PEDIATRIC Sterile Solution with Sodium Chloride Injection USP or Dextrose Injection USP. Undiluted PROSTIN VR PEDIATRIC Sterile Solution may interact with the plastic sidewalls of volumetric infusion chambers causing a change in the appearance of the chamber and creating a hazy solution. Should this occur, the solution and the volumetric infusion chamber should be replaced.
When using a volumetric infusion chamber, the appropriate amount of intravenous infusion solution should be added to the chamber first. The undiluted PROSTIN VR PEDIATRIC Sterile Solution should then be added to the intravenous infusion solution, avoiding direct contact of the undiluted solution with the walls of the volumetric infusion chamber.
Dilute to volumes appropriate for the pump delivery system available. Prepare fresh infusion solutions every 24 hours. Discard any solution more than 24 hours old.
| Add 1 ampoule (500 micrograms) alprostadil to: | Approximate Concentration of resulting solution (micrograms/mL) | Infusion rate (mL/min per kg of body weight) |
|---|---|---|
| Example: To provide 0.1 micrograms/kilogram of body weight per minute to an infant weighing 2.8 kilograms using a solution of 1 ampoule PROSTIN VR PEDIATRIC in 100 mL of saline or dextrose: INFUSION RATE = 0.02 mL/min per kg × 2.8 kg = 0.056 mL/min or 3.36 mL/hr. | ||
| 250 mL | 2 | 0.05 |
| 100 mL | 5 | 0.02 |
| 50 mL | 10 | 0.01 |
| 25 mL | 20 | 0.005 |
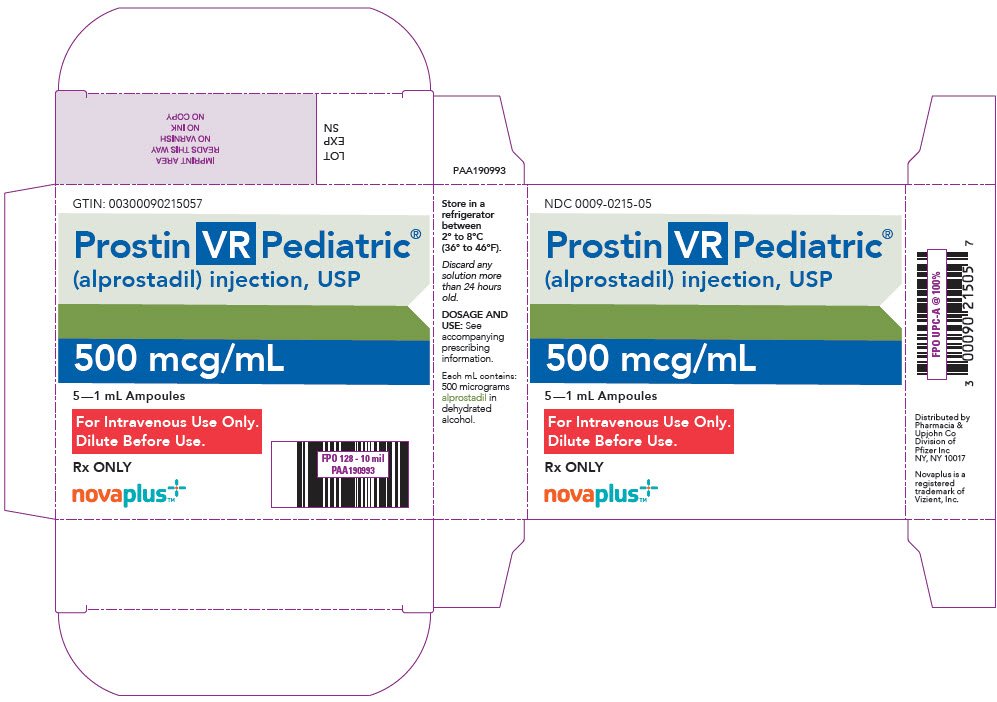
How is Prostin VR Pediatric supplied
PROSTIN VR PEDIATRIC Sterile Solution is available in a package of 5 ×1 mL ampoules (NDC 0009-0215-05). Each mL contains 500 micrograms alprostadil in dehydrated alcohol.
Rx only
Distributed by
Pharmacia & Upjohn Co
Division of Pfizer Inc
New York, NY 10017

Novaplus is a registered trademark of Vizient, Inc.
LAB-1522-1.0
Revised March 2022
PRINCIPAL DISPLAY PANEL - 1 mL Ampoule Label
NDC 0009-0215-01
Rx ONLY
Prostin VR Pediatric®
(alprostadil) injection, USP
For Intravenous Use Only.
Dilute Before Use.
1 mL
500 mcg/mL
Refrigerate at 2° to 8°C (36° to 46°F).
Distributed by Pharmacia & Upjohn Co
Division of Pfizer Inc,
NY, NY 10017
novaplus™
PAA190991
LOT
EXP

| PROSTIN
VR PEDIATRIC
alprostadil injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pharmacia & Upjohn Company LLC (618054084) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia & Upjohn Company LLC | 618054084 | ANALYSIS(0009-0215) , MANUFACTURE(0009-0215) , API MANUFACTURE(0009-0215) , PACK(0009-0215) , LABEL(0009-0215) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Manufacturing Belgium NV | 370156507 | ANALYSIS(0009-0215) , MANUFACTURE(0009-0215) , PACK(0009-0215) | |
More about Prostin VR Pediatric (alprostadil)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- Dosage information
- During pregnancy
- Generic availability
- Drug class: impotence agents
- En español
Patient resources
- Prostin VR Pediatric injectable and transurethral drug information
- Prostin VR Pediatric (Advanced Reading)
Professional resources
Other brands
Caverject, Muse, Edex, Caverject Impulse