Pertzye: Package Insert / Prescribing Info
Package insert / product label
Generic name: pancrelipase
Dosage form: capsule, delayed release
Drug class: Digestive enzymes
Medically reviewed by Drugs.com. Last updated on Mar 18, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
- Medication Guide
Highlights of Prescribing Information
PERTZYE (pancrelipase) delayed-release capsules, for oral use
Initial U.S. Approval: 2012
Indications and Usage for Pertzye
PERTZYE® is indicated for the treatment of exocrine pancreatic insufficiency in adult and pediatric patients. (1)
Pertzye Dosage and Administration
Important Dosing Information (2.1)
- PERTZYE is a mixture of enzymes including lipases, proteases, and amylases and dosing is based on lipase units. Dosing scheme based on actual body weight or fat ingestion.
- Individualize the dosage based on clinical symptoms, the degree of steatorrhea present, and the fat content of the diet.
- Do not exceed 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/g fat ingested/day in adult and pediatric patients greater than 12 months of age without further investigation. (5.1)
- The total daily dosage in adult and pediatric patients greater than 12 months of age should reflect approximately three meals plus two or three snacks per day. With each snack, administer approximately half the prescribed dose for a meal.
- Do not substitute other pancreatic enzyme products for PERTZYE. When switching from another pancreatic enzyme product to PERTZYE, monitor patients for clinical symptoms of exocrine pancreatic insufficiency and titrate the dosage as needed.
Recommended Dosage (2.2)
Adults and Pediatric Patients Greater than 12 Months: The recommended initial starting dosage is:
- 500 lipase units/kg/meal for adult and pediatric patients 4 years of age and older.
- 1,000 lipase units/kg/meal for pediatric patients greater than 12 months to less than 4 years of age.
- Titrate the dosage to either 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/g fat ingested/day. Higher dosages may be administered if documented to be effective by fecal fat measures or improvement in malabsorption.
Pediatric Patients Birth to 12 Months: The recommended dosage is 4,000 lipase units (one capsule) per 120 mL of formula or per breastfeeding.
Preparation and Administration Instructions (2.3)
- Swallow capsules whole. For patients unable to swallow intact capsule(s), the capsule contents may be sprinkled on soft acidic food (e.g., applesauce).
- The 4,000 USP lipase unit capsule may be administered with applesauce via gastrostomy tube (14 French or larger). The contents of no more than two capsules may be administered at a time.
- Do not crush or chew PERTZYE capsules or capsule contents.
- Consume sufficient liquids to ensure complete swallowing of PERTZYE. (5.2)
- See the full prescribing information for additional information on administering to pediatric patients birth to 12 months of age.
Dosage Forms and Strengths
Delayed-Release Capsules (3):
- 4,000 USP units of lipase; 14,375 USP units of protease; and 15,125 USP units of amylase.
- 8,000 USP units of lipase; 28,750 USP units of protease; and 30,250 USP units of amylase.
- 16,000 USP units of lipase; 57,500 USP units of protease; and 60,500 USP units of amylase.
- 24,000 USP units of lipase; 86,250 USP units of protease; and 90,750 USP units of amylase.
Contraindications
None. (4)
Warnings and Precautions
- Fibrosing Colonopathy: Associated with high doses, usually over prolonged use and in pediatric patients with cystic fibrosis. Colonic stricture reported in pediatric patients less than 12 years of age with dosages exceeding 6,000 lipase units/kg/meal. Monitor during treatment for progression of preexisting disease. Do not exceed the recommended dosage, unless clinically indicated. (2.1, 5.1)
- Irritation of the Oral Mucosa: May occur due to loss of protective enteric coating on the capsule contents. (5.2)
- Hyperuricemia: Reported with high dosages, consider monitoring blood uric acid levels in patients with gout, renal impairment, or hyperuricemia. (5.3)
- Risk of Viral Transmission: The presence of porcine viruses that might infect humans cannot be definitely excluded. (5.4)
- Hypersensitivity Reactions: Monitor patients with known reactions to proteins of porcine origin. If symptoms occur, initiate appropriate medical management; consider the risks and benefits of continued treatment. (5.5)
Adverse Reactions/Side Effects
Most common adverse reactions (≥ 10%) are: diarrhea, dyspepsia, and cough. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Digestive Care Inc. at 1-877-882-5950 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2024
Full Prescribing Information
1. Indications and Usage for Pertzye
PERTZYE® is indicated for the treatment of exocrine pancreatic insufficiency in adult and pediatric patients.
2. Pertzye Dosage and Administration
2.1 Important Dosing Information
PERTZYE is a mixture of enzymes including lipases, proteases, and amylases. PERTZYE dosing is based on lipase units.
- Use either an actual body weight or fat ingestion-based dosing scheme.
- Start at the lowest recommended dosage and individualize the dosage based on clinical symptoms, the degree of steatorrhea present, and the fat content of the diet. Changes in dosage may require an adjustment period of several days.
- Do not exceed 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/g fat ingested/day in adult and pediatric patients greater than 12 months of age without further investigation [see Warnings and Precautions (5.1)].
- The total daily dosage in adult and pediatric patients greater than 12 months of age should reflect approximately three meals plus two or three snacks per day. With each snack, administer approximately half the prescribed PERTZYE dose for a meal.
- Do not substitute other pancreatic enzyme products for PERTZYE. When switching from another pancreatic enzyme product to PERTZYE, monitor patients for clinical symptoms of exocrine pancreatic insufficiency and titrate the dosage as needed.
2.2 Recommended Dosage
Adults and Pediatric Patients Greater than 12 Months of Age
The recommended oral initial starting dosage is:
- 500 lipase units/kg/meal for adult and pediatric patients 4 years of age and older.
- 1,000 lipase units/kg/meal for pediatric patients greater than 12 months to less than 4 years of age.
If signs and symptoms of malabsorption persist, increase the dosage. Titrate to either 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/gram of fat ingested/day. Higher dosages may be administered if they are documented to be effective by fecal fat measures or an improvement in signs or symptoms of malabsorption including measures of nutritional status.
2.3 Preparation and Administration Instructions
Adult and Pediatric Patients Greater than 12 Months of Age
Instruct adult and pediatric patients greater than 12 months of age, or their caregivers, of the following:
- Take PERTZYE with meals or snacks. If a dose is missed, take the next dose with the next meal or snack.
- Swallow capsules whole.
- For patients unable to swallow intact capsules, the capsule contents may be sprinkled on a small amount of soft acidic food with a pH of 4.5 or less (e.g., applesauce). The 4,000 USP lipase unit capsule may also be administered with applesauce via gastrostomy tube 14 French or larger.
- Do not crush or chew PERTZYE capsules or capsule contents.
- Consume sufficient liquids (water or juice) to ensure complete swallowing of PERTZYE [see Warnings and Precautions (5.2)].
Oral Administration for Patients Unable to Swallow Intact Capsules
- Carefully open the capsules and sprinkle the entire contents on a small amount of acidic soft food (approximately 10 mL) with a pH of 4.5 or less (e.g., applesauce).
- Mix the capsule contents with the acidic soft food (e.g., applesauce) being careful not to crush the contents.
- Consume the entire mixture immediately. Do not save the mixture for later use.
- Consume sufficient fluids to ensure complete swallowing of the PERTZYE food mixture.
Gastrostomy Tube Administration (14 French Gastrostomy Tube or Larger)
- Only perform gastrostomy tube administration with the contents of the 4,000 USP lipase unit capsule of PERTZYE.
- The contents of no more than two capsules may be administered at a time in the gastrostomy tube.
- Transfer a minimum of 10 mL of applesauce into a small bowl or medicine cup.
- Carefully open one or two PERTZYE 4,000 lipase unit capsules.
- Mix the capsule contents thoroughly with the transferred applesauce to create a uniform suspension being careful not to crush the contents. Once mixed, administer the suspension immediately.
- Remove the plunger from a 35 mL slip tip syringe. Cover the tip of the syringe with your finger. Transfer the PERTZYE-applesauce mixture into the syringe. Replace the plunger partially back into the syringe.
- Shake or tap the syringe lightly with the syringe tip facing upward so that the PERTZYE-applesauce mixture will move towards the plunger. Carefully push the plunger slowly until the residual air is removed from the syringe tip.
- Once the residual air is removed, connect the syringe directly into the gastrostomy tube feeding port.
- Push the syringe contents into the gastrostomy tube feeding port using steady pressure until empty.
- Draw up approximately 10 mL of water with the slip tip syringe and flush the gastrostomy tube feeding port with the water.
- Discard any unused portion of the PERTZYE-applesauce mixture. Do not save for later use.
- If dose requires more than two capsules, repeat steps 1-9 until prescribed dose is reached.
Pediatric Patients Birth to 12 Months of Age:
Instruct caregivers of pediatric patients birth to 12 months of age of the following:
- Immediately prior to each breastfeeding session or each administration of 120 mL of formula, carefully open one PERTZYE capsule (containing 4,000 USP units of lipase) and administer the entire contents using one of the following two methods:
- Sprinkle on a small amount of acidic soft food with a pH of 4.5 or less (e.g., applesauce) being careful not to crush the capsule contents. The entire mixture should be given to the infant immediately.
- Sprinkle the capsule contents directly into the infant's mouth.
- Immediately administer breast milk or formula after PERTZYE to ensure complete swallowing of the PERTZYE capsule contents.
- Do not crush PERTZYE capsule contents, and visually inspect the infant's mouth to ensure that no drug is retained in the mouth [see Warnings and Precautions (5.2)].
- If a dose is missed, administer the next dose with the next feeding.
3. Dosage Forms and Strengths
Delayed-release capsules are available in the following strengths:
- 4,000 USP units of lipase; 14,375 USP units of protease; and 15,125 USP units of amylase in a two-piece hard gelatin capsule with a clear body printed in green with "4" and a clear cap printed with a green circular stripe and "DCI"
- 8,000 USP units of lipase; 28,750 USP units of protease; and 30,250 USP units of amylase in a two-piece hard gelatin capsule with a clear body printed in blue with "8" and a clear cap printed with a blue circular stripe and "DCI"
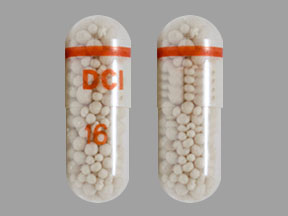
- 16,000 USP units of lipase; 57,500 USP units of protease; and 60,500 USP units of amylase in a two-piece hard gelatin capsule with a clear body printed in red with "16" and a clear cap printed with a red circular stripe and "DCI"
- 24,000 USP units of lipase; 86,250 USP units of protease; and 90,750 USP units of amylase in a two-piece hard gelatin capsule with a clear body printed in purple with "24" and a clear cap printed with a purple circular stripe and "DCI"
5. Warnings and Precautions
5.1 Fibrosing Colonopathy
Fibrosing colonopathy has been reported following treatment with pancreatic enzyme products. Fibrosing colonopathy is a rare, serious adverse reaction initially described in association with use of high-dose pancreatic enzyme products, usually with use over a prolonged period of time and most commonly reported in pediatric patients with cystic fibrosis. Pancreatic enzyme products exceeding 6,000 lipase units/kg/meal have been associated with colonic stricture, a complication of fibrosing colonopathy, in pediatric patients less than 12 years of age. The underlying mechanism of fibrosing colonopathy remains unknown.
If there is a history of fibrosing colonopathy, monitor patients during treatment with PERTZYE because some patients may be at risk of progressing to colonic stricture formation. It is uncertain whether regression of fibrosing colonopathy occurs. Do not exceed the recommended dosage of either 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/g fat ingested/day in adult and pediatric patients greater than 12 months of age without further investigation. Higher dosages may be administered if they are documented to be effective by fecal fat measures or an improvement in signs and symptoms of malabsorption including measures of nutritional status. Patients receiving dosages higher than 6,000 lipase units/kg/meal should be frequently monitored for symptoms of fibrosing colonopathy and the dosage decreased or titrated downward to a lower range if clinically appropriate [see Dosage and Administration (2.1)].
5.2 Irritation of the Oral Mucosa
Crushing or chewing PERTZYE capsules or mixing the capsule contents in foods having a pH greater than 4.5 can disrupt the protective enteric coating on the capsule contents and result in early release of enzymes, irritation of the oral mucosa, and/or loss of enzyme activity.
Instruct the patient or caregiver of the following:
- Swallow capsules whole. For patients who cannot swallow the capsules whole, the capsules can be opened, and the contents sprinkled on a small amount of acidic soft food with a pH of 4.5 or less (e.g., applesauce). The 4,000 USP lipase unit capsule may also be administered with applesauce via a gastrostomy tube with a diameter of 14 French or larger.
- Do not crush or chew PERTZYE capsules or capsule contents.
- Consume sufficient liquids (juice, water, breast milk, or formula) immediately following administration of PERTZYE to ensure complete swallowing.
- Visually inspect the mouth of pediatric patients less than 12 months of age and of patients who are unable to swallow intact capsules to ensure no drug is retained in the mouth and irritation of the oral mucosa has not occurred [see Dosage and Administration (2.3)].
5.3 Hyperuricemia
Pancreatic enzyme products contain purines that may increase blood uric acid levels. High dosages have been associated with hyperuricosuria and hyperuricemia [see Overdosage (10)]. Consider monitoring blood uric acid levels in patients with gout, renal impairment, or hyperuricemia during treatment with PERTZYE.
5.4 Risk of Viral Transmission
PERTZYE is sourced from pancreatic tissue from swine used for food consumption. Although the risk that PERTZYE will transmit an infectious agent to humans has been reduced by testing for certain viruses during manufacturing and by inactivating certain viruses during manufacturing, there is a theoretical risk for transmission of viral disease, including diseases caused by novel or unidentified viruses. Thus, the presence of porcine viruses that might infect humans cannot be definitely excluded. However, no cases of transmission of an infectious illness associated with the use of porcine pancreatic extracts have been reported.
5.5 Hypersensitivity Reactions
Severe hypersensitivity reactions including anaphylaxis, asthma, hives, and pruritus have been reported with pancreatic enzyme products [see Adverse Reactions (6.2)]. If symptoms occur, initiate appropriate medical management.
Monitor patients with a known hypersensitivity reaction to proteins of porcine origin for hypersensitivity reactions during treatment with PERTZYE. The risks and benefits of continued PERTZYE treatment in patients with severe hypersensitivity reactions should be taken into consideration with the overall clinical needs of the patient.
6. Adverse Reactions/Side Effects
The following serious or otherwise important adverse reactions are described elsewhere in the labeling:
- Fibrosing Colonopathy [see Warnings and Precautions (5.1)]
- Irritation of the Oral Mucosa [see Warnings and Precautions (5.2)]
- Hyperuricemia [see Warnings and Precautions (5.3)]
- Risk of Viral Transmission [see Warnings and Precautions (5.4)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described below reflect exposure to PERTZYE in 21 patients, aged 8 to 43 years, with exocrine pancreatic insufficiency due to cystic fibrosis in a placebo-controlled clinical trial [see Clinical Studies (14)].
Table 1 enumerates adverse reactions that occurred in at least 2 patients (greater than or equal to 10%) treated with PERTZYE at a higher rate than with placebo.
| Adverse Reaction | PERTZYE N=21 (%) | Placebo N=24 (%) |
|---|---|---|
|
||
| Diarrhea | 10% | 4% |
| Dyspepsia | 10% | 4% |
| Cough | 10% | 4% |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of PERTZYE or other pancreatic enzyme products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Eye Disorders
- blurred vision
Gastrointestinal Disorders
- fibrosing colonopathy, distal intestinal obstruction syndrome
- abdominal pain, flatulence, constipation, and nausea
Immune System Disorders
- anaphylaxis, asthma, hives, and pruritus
Investigations
- asymptomatic elevations of liver enzymes
Musculoskeletal System
- myalgia, muscle spasm
Skin and Subcutaneous Tissue Disorders
- urticaria and rash
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Published data from case reports with pancrelipase use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. Pancrelipase is minimally absorbed systematically; therefore, maternal use is not expected to result in fetal exposure to the drug. Animal reproduction studies have not been conducted with pancrelipase.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of pancrelipase in either human or animal milk, the effects on the breastfed infant or the effects on milk production. Pancrelipase is minimally absorbed systemically following oral administration, therefore maternal use is not expected to result in clinically relevant exposure of breastfed infants to the drug. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for PERTZYE and any potential adverse effects on the breastfed infant from PERTZYE or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of PERTZYE for the treatment of exocrine pancreatic insufficiency have been established in pediatric patients.
Use of PERTZYE for this indication is supported by a randomized, double-blind, placebo-controlled, crossover study of 24 pediatric patients, 8 years and older with exocrine pancreatic insufficiency due to cystic fibrosis. The safety in pediatric patients was similar to that observed in adult patients [see Adverse Reactions (6.1) and Clinical Studies (14)].
Dosages exceeding 6,000 lipase units/kg/meal have been reported postmarketing to be associated with fibrosing colonopathy and colonic strictures in pediatric patients less than 12 years of age. If there is a history of fibrosing colonopathy, monitor patients during treatment with PERTZYE because some patients may be at risk of progressing to stricture formation. Do not exceed the recommended dosage of either 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/g fat ingested/day in pediatric patients greater than 12 months of age without further investigation [see Dosage and Administration (2.1) and Warnings and Precautions (5.1)].
Crushing or chewing PERTZYE capsules or mixing the capsule contents in foods having a pH greater than 4.5 can disrupt the protective enteric coating on the capsule contents and result in early release of enzymes, irritation of the oral mucosa, and/or loss of enzyme activity. Instruct the patient or caregiver of the following: consume sufficient liquids (juice, water, breast milk, or formula) to ensure complete swallowing, and visually inspect the mouth of pediatric patients less than 12 months of age to ensure that no drug is retained in the mouth and irritation of the oral mucosa has not occurred [see Dosage and Administration (2.3) and Warnings and Precautions (5.2)].
8.5 Geriatric Use
Clinical studies of PERTZYE did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between patients aged 65 years and over and younger adult patients.
10. Overdosage
Chronic high dosages of pancreatic enzyme products have been associated with fibrosing colonopathy and colonic strictures [see Warnings and Precautions (5.1)]. High dosages of pancreatic enzyme products have been associated with hyperuricosuria and hyperuricemia [see Warnings and Precautions (5.3)].
11. Pertzye Description
Pancrelipase is a pancreatic enzyme product consisting of a mixture of enzymes including lipases, proteases, and amylases, and is an extract derived from porcine pancreatic glands. The enteric-coated microspheres in PERTZYE are formulated to release pancreatic enzymes at an approximate pH of 5.5 or greater.
PERTZYE (pancrelipase) delayed-release capsules are for oral administration, include a two-piece hard gelatin shell containing light tan/cream-colored bicarbonate-buffered enteric-coated microspheres ranging in size from 0.8 to 1.4 mm in diameter for 4,000 USP units of lipase and 0.8 to 2.2 mm in diameter for 8,000, 16,000, and 24,000 USP units of lipase, and are available as follows:
4,000 USP units of lipase; 14,375 USP units of protease; and 15,125 USP units of amylase; delayed-release capsules have a clear body printed in green with "4" and a clear cap printed with a green circular stripe and "DCI". The imprinting ink on the capsule contains FD&C Blue #1, D&C Yellow #10, black iron oxide, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, shellac, and ammonium hydroxide.
8,000 USP units of lipase; 28,750 USP units of protease; and 30,250 USP units of amylase; delayed-release capsules have a clear body printed in blue with "8" and a clear cap printed with a blue circular stripe and "DCI". The imprinting ink on the capsule contains FD&C Blue #1, ethanol, methanol, n-butyl alcohol, propylene glycol, shellac, and ammonium hydroxide.
16,000 USP units of lipase; 57,500 USP units of protease; and 60,500 USP units of amylase; delayed-release capsules have a clear body printed in red with "16" and a clear cap printed with a red circular stripe and "DCI". The imprinting ink on the capsule contains FD&C Red #40, povidone, titanium dioxide, dehydrated alcohol, sodium hydroxide, butyl alcohol, propylene glycol, isopropyl alcohol, and shellac.
24,000 USP units of lipase; 86,250 USP units of protease; and 90,750 USP units of amylase; delayed-release capsules have a clear body printed in purple with "24" and a clear cap printed with a purple circular stripe and "DCI". The imprinting ink on the capsule contains FD&C Blue #2, D&C Red #7, titanium dioxide, strong ammonia solution, propylene glycol, butyl alcohol, isopropyl alcohol, dehydrated alcohol, and shellac.
PERTZYE (pancrelipase) delayed-release capsules include the following inactive ingredients: cellulose acetate phthalate, diethyl phthalate, polyvinylpyrrolidone, sodium bicarbonate, sodium carbonate, sodium starch glycolate, talc, and ursodiol.
12. Pertzye - Clinical Pharmacology
12.1 Mechanism of Action
Pancreatic enzyme products contain a mixture of lipases, proteases, and amylases that catalyze the hydrolysis of fats to monoglyceride, glycerol and free fatty acids, proteins into peptides and amino acids, and starches into dextrins and short chain sugars such as maltose and maltotriose in the duodenum and proximal small intestine, thereby acting like digestive enzymes physiologically secreted by the pancreas.
12.2 Pharmacodynamics
For patients consuming a high fat diet in the clinical trials, the coefficient of fat absorption (CFA) was higher in patients who received PERTZYE compared to the placebo treatment group, indicating improved fat absorption [see Clinical Studies (14)].
14. Clinical Studies
Adult and Pediatric Patients
A randomized, double-blind, placebo-controlled, crossover study was conducted in 24 patients aged 8 to 43 years (mean age of 20 years) with exocrine pancreatic insufficiency due to cystic fibrosis. Patients were randomized to receive PERTZYE at individually titrated doses (not to exceed 2,500 lipase units/kg/meal) or matching placebo for 6 to 8 days of treatment, followed by crossover to the alternate treatment for an additional 6 to 8 days. The mean lipase dosage during the treatment period was 1,565 lipase units/kg/meal and a controlled high-fat diet (approximately 2g fat/kg/day) was maintained throughout the study. The length of exposure to PERTZYE during this study was 20 to 28 days, including the treatment period of 6 to 8 days, and the open label titration and transition periods of 7 to 10 days each.
The efficacy analysis population included 21 patients who completed both double-blind treatment periods.
Coefficient of Fat Absorption Endpoint and Results
The primary efficacy endpoint was the mean difference in coefficient of fat absorption (CFA) between PERTZYE and placebo treatment. The CFA was determined by a 72-hour stool collection during both treatments, when both fat ingestion and excretion were measured.
Mean CFA was 83% with PERTZYE treatment compared to 46% with placebo treatment. The mean difference in CFA was 36 percentage points in favor of PERTZYE treatment with 95% CI: (28, 45) and p<0.001.
Coefficient of Nitrogen Absorption Endpoint and Results
The coefficient of nitrogen absorption (CNA) was determined by a 72-hour stool collection during both treatments, when nitrogen excretion was measured and nitrogen ingestion from a controlled diet was estimated (based on the assumption that proteins contain 16% nitrogen). Each patient's CNA during placebo treatment was used as their no-treatment CNA value.
Mean CNA was 79% with PERTZYE treatment compared to 47% with placebo treatment. The mean difference in CNA was 32 percentage points in favor of PERTZYE treatment and this was a statistically significant change.
There were no differences between pediatric patients and adults in the severity of pancreatic insufficiency (placebo response) or in the magnitude of the response to PERTZYE.
16. How is Pertzye supplied
PERTZYE (pancrelipase) delayed-release capsules, containing light tan/cream-colored delayed-release microspheres are supplied as follows:
| Strength | Description | Supplied As | NDC Number |
|---|---|---|---|
| 4,000 USP units of lipase; 14,375 USP units of protease; 15,125 USP units of amylase | Two-piece hard gelatin capsule with a clear body printed in green with "4" and a clear cap printed with a green circular stripe and "DCI" | Bottles of 100 | 59767-004-01 |
| 8,000 USP units of lipase; 28,750 USP units of protease; 30,250 USP units of amylase | Two-piece hard gelatin capsule with a clear body printed in blue with "8" and a clear cap printed with a blue circular stripe and "DCI" | Bottles of 100 Bottles of 250 | 59767-008-01 59767-008-02 |
| 16,000 USP units of lipase; 57,500 USP units of protease; 60,500 USP units of amylase | Two-piece hard gelatin capsule with a clear body printed in red with "16" and a clear cap printed with a red circular stripe and "DCI" | Bottles of 100 Bottles of 250 | 59767-016-01 59767-016-02 |
| 24,000 USP units of lipase; 86,250 USP units of protease; 90,750 USP units of amylase | Two-piece hard gelatin capsule with a clear body printed in purple with "24" and a clear cap printed with a purple circular stripe and "DCI" | Bottles of 80 Bottles of 200 | 59767-024-01 59767-024-02 |
Storage and Handling
- Store PERTZYE at room temperature 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 40°C (59°F to 104°F) for up to 30 days. PERTZYE hard gelatin capsules should be stored in a dry place in the original container or equivalent airtight container. After opening, keep the container tightly closed between uses to protect from moisture.
- PERTZYE is dispensed in bottles containing a desiccant.
17. Patient Counseling Information
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Fibrosing Colonopathy
Advise the patient or caregiver that fibrosing colonopathy has been reported with high dosages of pancreatic enzyme products, usually with use over a prolonged period of time and in pediatric patients less than 12 years of age. Advise patients and caregivers that if signs and symptoms of colon stricture formation occur (e.g., stomach area (abdominal) pain, bloating, trouble passing stool (constipation), nausea, vomiting, diarrhea) to immediately contact their healthcare provider [see Warnings and Precautions (5.1)].
Hyperuricemia
Advise the patient or caregiver that hyperuricemia may occur in patients with gout or renal impairment and to contact the healthcare provider if they experience pain, stiffness, redness or swelling of their joints [see Warnings and Precautions (5.3)].
Hypersensitivity Reactions
Inform the patient or caregiver that severe hypersensitivity reactions, including anaphylaxis, asthma, hives, and pruritus, have been reported with use of pancreatic enzyme products. Seek medical attention if signs or symptoms of a hypersensitivity reaction develop [see Warnings and Precautions (5.5)].
Dosage
Advise the patient or caregiver to take or administer PERTZYE as prescribed, and to contact the healthcare provider if signs and symptoms of malabsorption persist [see Dosage and Administration (2.2)].
Administration
Instruct the patient or caregiver to:
- Take PERTZYE with meals or snacks.
- Swallow capsules whole.
- For patients unable to swallow intact capsules:
- the capsule contents may be sprinkled on a small amount of soft acidic food with a pH of 4.5 or less (e.g., applesauce). The 4,000 USP lipase unit capsule may also be administered with applesauce via gastrostomy tube 14 French or larger.
- For pediatric patients birth to 12 months of age, PERTZYE capsules can be opened, and the capsule contents sprinkled directly into the infant's mouth.
- See the Instructions for Use for a description of all preparation and administration instructions.
- Consume sufficient liquids (juice, water, breast milk, or formula) and visually inspect an infant's mouth to ensure complete swallowing of PERTZYE capsules or capsule contents [see Warnings and Precautions (5.2)].
- Do not crush or chew PERTZYE capsules or capsule contents.
- Do not mix the PERTZYE capsule contents directly into a bottle of breast milk or formula.
Manufactured by:
Digestive Care, Inc.
1120 Win Drive
Bethlehem, PA 18017
U.S. License No. 2184
Product of USA
PERTZYE® is a registered trademark of Digestive Care, Inc.
U.S. Patent Numbers: 5,260,074; 5,302,400; 5,324,514; 5,460,812; 5,578,304; 5,750,104
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: 02/2024 | ||
| MEDICATION GUIDE PERTZYE (pert-zye) (pancrelipase) delayed-release capsules |
|||
| What is the most important information I should know about PERTZYE?
PERTZYE may increase the risk of having a rare bowel disorder called fibrosing colonopathy, especially if taken at a high dose for a long time in children with cystic fibrosis. This condition is serious and may require surgery. The risk of having this condition may be reduced by following the dosing instructions that your doctor gives you. Call your doctor right away if you have any unusual or severe: |
|||
|
|
||
| Take PERTZYE exactly as prescribed by your doctor. Do not take more PERTZYE than directed by your doctor. | |||
What is PERTZYE?
|
|||
Before taking PERTZYE, tell your doctor about all of your medical conditions, including if you:
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine. |
|||
How should I take PERTZYE?
|
|||
What are the possible side effects of PERTZYE?
|
|||
| The most common side effects of PERTZYE include: | |||
|
|
||
| Other possible side effects:
PERTZYE and other pancreatic enzyme products are made from the pancreas of pigs, the same pigs people eat as pork. These pigs may carry viruses. Although it has never been reported, it may be possible for a person to get a viral infection from taking pancreatic enzyme products that come from pigs. Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of PERTZYE. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
How should I store PERTZYE?
|
|||
| General information about the safe and effective use of PERTZYE.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use PERTZYE for a condition for which it was not prescribed. Do not give PERTZYE to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about PERTZYE that is written for health professionals. |
|||
| What are the ingredients in PERTZYE?
Active ingredients: lipase, protease, and amylase. Inactive ingredients: cellulose acetate phthalate, diethyl phthalate, polyvinylpyrrolidone, sodium bicarbonate, sodium carbonate, sodium starch glycolate, talc, and ursodiol. The hard gelatin capsule shells contain: gelatin and water. The green imprinting ink on the 4,000 units of lipase; 14,375 units of protease; 15,125 units of amylase capsules contain: FD&C Blue #1, D&C Yellow #10, black iron oxide, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, shellac and ammonium hydroxide. The blue imprinting ink on the 8,000 units of lipase; 28,750 units of protease; 30,250 units of amylase capsules contain: FD&C Blue #1, ethanol, methanol, n-butyl alcohol, propylene glycol, shellac and ammonium hydroxide. The red imprinting ink on the 16,000 units of lipase; 57,500 units of protease; 60,500 units of amylase capsules contain: FD&C Red #40, povidone, titanium dioxide, dehydrated alcohol, sodium hydroxide, butyl alcohol, propylene glycol, isopropyl alcohol, and shellac. The purple imprinting ink on the 24,000 units of lipase; 86,250 units of protease; 90,750 units of amylase capsules contain: FD&C Blue #2, D&C Red #7, titanium dioxide, strong ammonia solution, propylene glycol, butyl alcohol, isopropyl alcohol, dehydrated alcohol, and shellac. For more information, go to www.Pertzye.com or call 1-877-882-5950. |
|||
| Manufactured by:
Digestive Care, Inc. 1120 Win Drive Bethlehem, PA 18017 U.S. License No. 2184 Product of USA |
|||
INSTRUCTIONS FOR USE
PERTZYE (pert-zye)
(pancrelipase)
delayed-release capsules
This Instructions for Use contains information on how to give PERTZYE by mouth and through a gastrostomy tube.
Important Information You Need to Know Before Taking PERTZYE:
- Always take PERTZYE with a meal or snack.
- PERTZYE capsules should be swallowed whole. Do not crush or chew the PERTZYE capsules or their contents, and do not hold the capsule or capsule contents in your mouth.
- Drink plenty of liquid to ensure complete swallowing of PERTZYE capsules or PERTZYE capsule contents-food mixture.
- Throw away the PERTZYE capsule contents-food mixture that is not used. Do not save this mixture for later use.
Giving PERTZYE to infants (Children from Birth to 12 Months of Age) by mouth:
The two methods to give PERTZYE to Infants (Children from Birth to 12 Months of Age) are described below:
a) Giving with Applesauce Before Breast or Formula Feeding
- Place about 2 teaspoons of applesauce, the kind found in baby food jars that you buy at the store, or other food recommended by your doctor, into a clean container.
- Carefully open the PERTZYE capsule by grasping and slightly squeezing both ends of the capsule and then twisting and pulling the capsule apart. Pour the capsule contents onto the food in the clean container. Throw away the empty PERTZYE capsule.
- Carefully mix the capsule contents evenly through the food. Be careful not to crush the PERTZYE capsule contents when mixing.
- Give the PERTZYE capsule contents-food mixture to your infant right away.
- Give your infant 4 ounces (120 mL) of formula or breastfeed your infant right away.
- Look in your infant's mouth to make sure that all of the medicine is swallowed and there is no PERTZYE capsule contents-food mixture left in your infant's mouth.
b) Giving Directly into the Infant's Mouth Before Breast or Formula Feeding
- Carefully open the PERTZYE capsule by grasping and slightly squeezing both ends of the capsule and then twisting and pulling the capsule apart. Empty the capsule contents into your infant's mouth.
- Give your infant 4 ounces (120 mL) of formula or breastfeed right away.
- Look in your infant's mouth to make sure that all of the medicine is swallowed and there is no PERTZYE capsule contents left in your infant's mouth.
- Throw away the empty PERTZYE capsule.
Do not mix the PERTZYE capsule contents directly into a bottle of breast milk or formula.
Giving PERTZYE to children and adults by mouth:
- Swallow PERTZYE capsules whole and take them with enough liquid to swallow them right away.
- If you have trouble swallowing capsules, follow these instructions for taking PERTZYE with food:
- Place about 2 teaspoons of applesauce, or other food recommended by your doctor, into a clean container.
- Carefully open the capsules that you will need for the prescribed dose by grasping and slightly squeezing both ends of the capsule and then twisting and pulling the capsule apart. Pour the capsule contents onto the food in the clean container.
- Throw away the empty PERTZYE capsules.
- Carefully mix the capsule contents evenly through the food. Be careful not to crush the PERTZYE capsule contents when mixing.
- Swallow all of the PERTZYE capsule contents-food mixture right away.
- Drink plenty of water or juice to make sure that all of the medicine is swallowed and there is no PERTZYE capsule contents-food mixture left in the mouth.
Giving PERTZYE through a gastrostomy tube:
- PERTZYE 4,000 lipase units capsule contents may be given through a gastrostomy tube that is size 14 French or larger. Do not give PERTZYE through a gastrostomy tube smaller than size 14 French.
- Do not give more than the contents of 2 PERTZYE 4,000 lipase units capsules through a gastrostomy tube at a time.
- Give the PERTZYE capsule contents-food mixture right away after it is prepared.
- 1.
- Place at least 2 teaspoons of applesauce, or other food recommended by your doctor, into a clean container.
- 2.
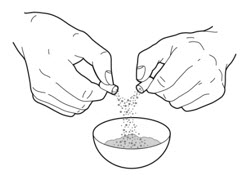
- Carefully open 1 or 2 of the PERTZYE capsules, depending on your prescribed dose, by grasping and slightly squeezing both ends of the capsule and then twisting and pulling the capsule apart. Pour the capsule contents onto the food in the clean container. Throw away the empty PERTZYE capsules (See Figure A).
- 3.
- Carefully mix the capsule contents evenly through the food. Be careful not to crush the PERTZYE capsule contents when mixing.
- 4.
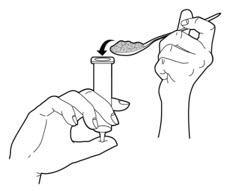
- Remove the plunger from a 35 mL slip tip syringe. Cover the tip of the syringe with your finger.
- 5.
- Carefully spoon the PERTZYE capsule contents-food mixture into the slip tip syringe (See Figure B).
- 6.
- Replace the plunger partially back into the slip tip syringe.
- 7.
- Turn the slip tip syringe so the syringe tip is facing upward. Remove your finger from the tip of the slip tip syringe.
- 8.
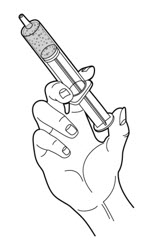
- Gently shake or tap the slip tip syringe so that the PERTZYE capsule contents-food mixture moves down towards the plunger.
- 9.
- Carefully push up slowly on the plunger until the PERTZYE capsule contents-food mixture fills the syringe tip (See Figure C).
- 10.
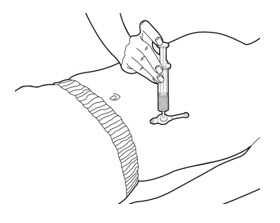
- Connect the slip tip syringe directly into the gastrostomy tube feeding port.
- 11.
- Press on the plunger of the slip tip syringe using steady pressure to push all of the PERTZYE capsule contents-food mixture into the gastrostomy tube feeding port over 10 seconds to 12 seconds (See Figure D).
- 12.
- Flush the gastrostomy tube feeding port with about 10 mL of water.
- 13.
- If your prescribed dose is more than 2 capsules of PERTZYE, repeat steps 1 through 12, using only 1 or 2 PERTZYE capsules at a time, until the prescribed dose is given.
Storing PERTZYE:
- Store PERTZYE at room temperature between 68ºF to 77ºF (20°C to 25°C).
- Keep PERTZYE in a dry place and in the original container or equivalent airtight container.
- After opening the bottle, keep it tightly closed between uses to keep your medicine dry (protect it from moisture).
- The PERTZYE bottle contains a desiccant packet to help keep your medicine dry. Do not eat or throw away the desiccant packet in your medicine bottle.
Keep PERTZYE and all medicines out of the reach of children.
Manufactured by:
Digestive Care, Inc.
1120 Win Drive
Bethlehem, PA 18017
U.S. License No. 2184
Product of USA
© 2024 Digestive Care, Inc.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 02/2024
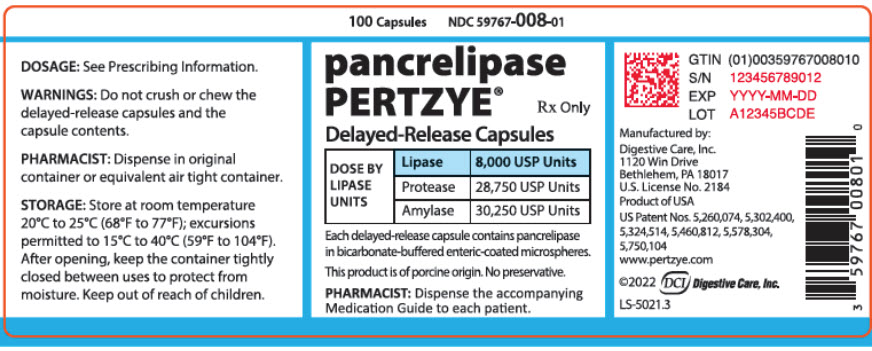
PRINCIPAL DISPLAY PANEL - 100 Capsule Bottle Label - 59767-008-01
100 Capsules
NDC 59767-008-01
pancrelipase
PERTZYE®
Rx Only
Delayed-Release Capsules
DOSE BY
LIPASE
UNITS
Lipase
8,000 USP Units
Protease
28,750 USP Units
Amylase
30,250 USP Units
Each delayed-release capsule contains pancrelipase
in bicarbonate-buffered enteric-coated microspheres.
This product is of porcine origin. No preservative.
PHARMACIST: Dispense the accompanying
Medication Guide to each patient.
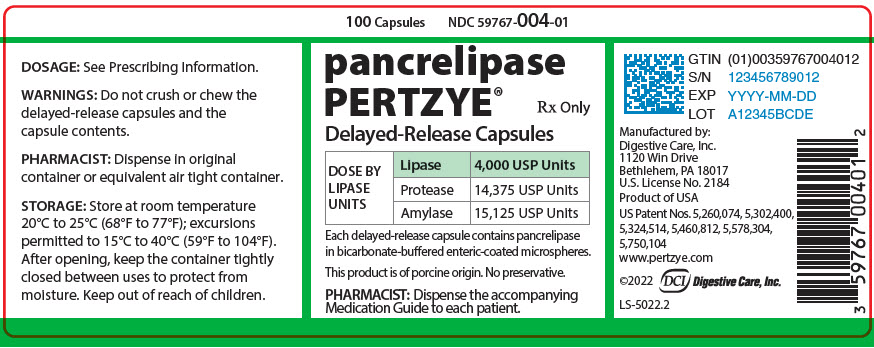
PRINCIPAL DISPLAY PANEL - 100 Capsule Bottle Label - 59767-004-01
100 Capsules
NDC 59767-004-01
pancrelipase
PERTZYE®
Rx Only
Delayed-Release Capsules
DOSE BY
LIPASE
UNITS
Lipase
4,000 USP Units
Protease
14,375 USP Units
Amylase
15,125 USP Units
Each delayed-release capsule contains pancrelipase
in bicarbonate-buffered enteric-coated microspheres.
This product is of porcine origin. No preservative.
PHARMACIST: Dispense the accompanying
Medication Guide to each patient.
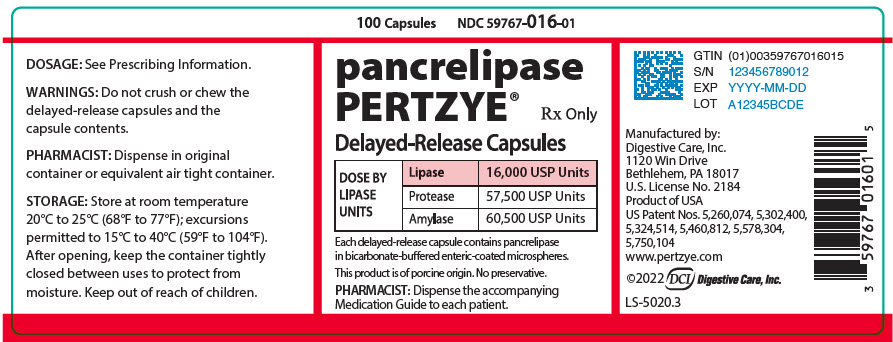
PRINCIPAL DISPLAY PANEL - 100 Capsule Bottle Label - 59767-016-01
100 Capsules
NDC 59767-016-01
pancrelipase
PERTZYE®
Rx Only
Delayed-Release Capsules
DOSE BY
LIPASE
UNITS
Lipase
16,000 USP Units
Protease
57,500 USP Units
Amylase
60,500 USP Units
Each delayed-release capsule contains pancrelipase
in bicarbonate-buffered enteric-coated microspheres.
This product is of porcine origin. No preservative.
PHARMACIST: Dispense the accompanying
Medication Guide to each patient.
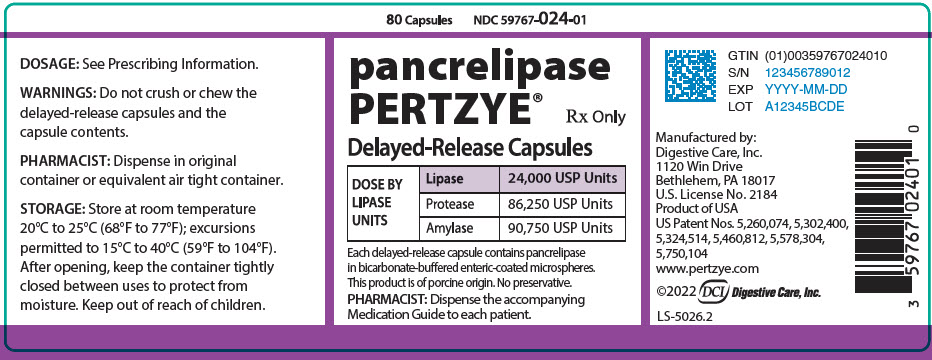
PRINCIPAL DISPLAY PANEL - 80 Capsule Bottle Label
80 Capsules
NDC 59767-024-01
pancrelipase
PERTZYE®
Rx Only
Delayed-Release Capsules
DOSE BY
LIPASE
UNITS
Lipase
24,000 USP Units
Protease
86,250 USP Units
Amylase
90,750 USP Units
Each delayed-release capsule contains pancrelipase
in bicarbonate-buffered enteric-coated microspheres.
This product is of porcine origin. No preservative.
PHARMACIST: Dispense the accompanying
Medication Guide to each patient.
| PERTZYE
pancrelipase capsule, delayed release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| PERTZYE
pancrelipase capsule, delayed release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PERTZYE
pancrelipase capsule, delayed release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| PERTZYE
pancrelipase capsule, delayed release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Digestive Care, Inc. (831065214) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Digestive Care, Inc. | 831065214 | manufacture(59767-008, 59767-016, 59767-004, 59767-024) | |
Frequently asked questions
More about Pertzye (pancrelipase)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: digestive enzymes
- Breastfeeding
- En español
Patient resources
Professional resources
Other brands
Creon, Zenpep, Pancreaze, Viokace