Neuraceq: Package Insert / Prescribing Info
Package insert / product label
Generic name: florbetaben f 18
Dosage form: injection, solution
Drug class: Diagnostic radiopharmaceuticals
Medically reviewed by Drugs.com. Last updated on Jul 2, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
NEURACEQ (florbetaben F 18 injection), for intravenous use
Initial U.S. Approval: 2014
Recent Major Changes
Indications and Usage (1) 6/2025
Dosage and Administration (2), 6/2025
Image Display and Interpretation (2.4)
Indications and Usage for Neuraceq
NEURACEQ is a radioactive diagnostic drug indicated for positron emission tomography (PET) of the brain to estimate amyloid beta neuritic plaque density in adults with cognitive impairment for: (1)
- Evaluation of Alzheimer’s disease (AD) and other causes of cognitive decline
- Selection of patients who are indicated for amyloid beta-directed therapy as described in the prescribing information of the therapeutic products (1)
Neuraceq Dosage and Administration
- The recommended amount of radioactivity is 300 MBq (8.1 mCi) administered as a slow single intravenous bolus (6 sec/mL) in a total volume of up to 10 mL. (2.2)
- Follow the injection with an intravenous flush of approximately 10 mL of 0.9% sodium chloride injection. (2.2)
- Obtain 15-minute to 20-minute PET images starting approximately 45 minutes to 130 minutes after drug administration. (2.3)
- See full prescribing information for image interpretation and radiation dosimetry. (2.4, 2.5)
Dosage Forms and Strengths
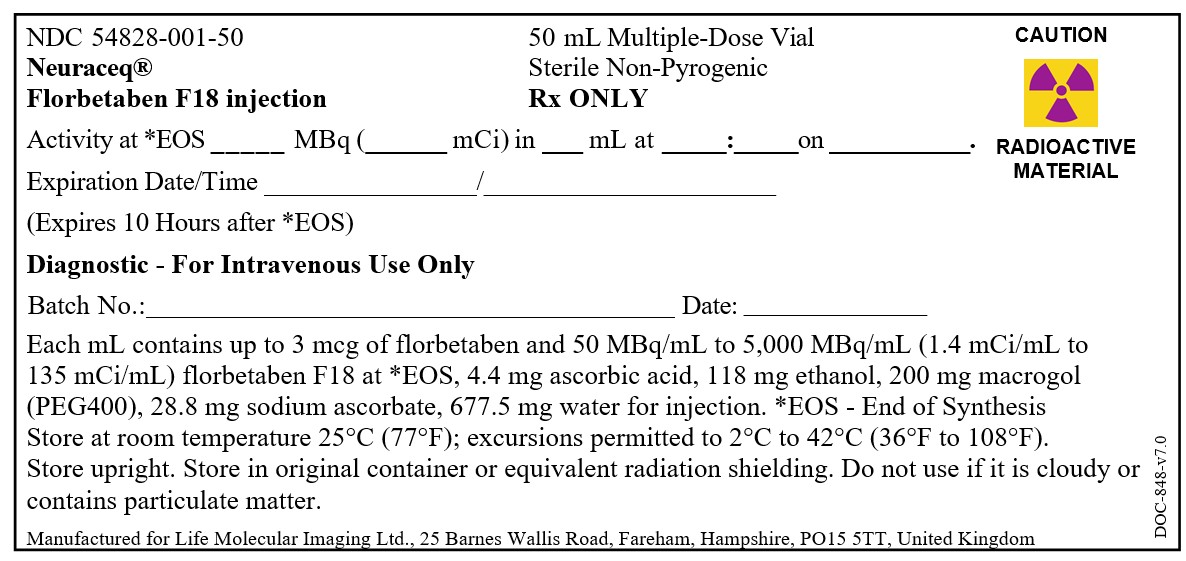
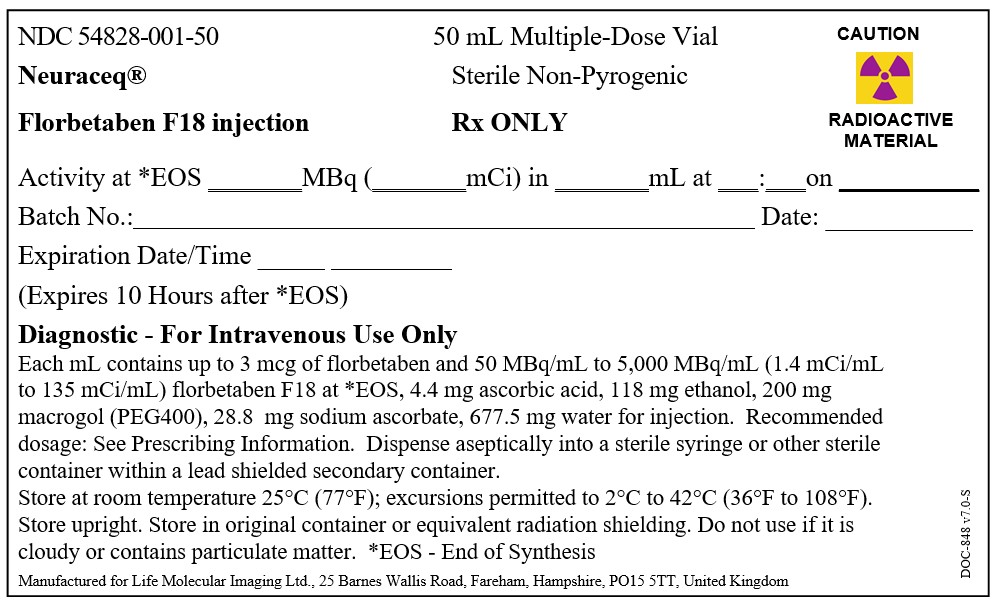
Injection: 50 MBq/mL to 5,000 MBq/mL (1.4 mCi/mL to 135 mCi/mL) of florbetaben F 18 in up to 50 mL volume at end of synthesis in a multiple-dose vial (3)
Contraindications
None. (4)
Warnings and Precautions
- Risk of Image Misinterpretation and Other Errors: Image interpretation errors have been observed. (5.1)
- Radiation Risk: NEURACEQ contributes to a patient’s long-term cumulative radiation exposure. Ensure safe drug handling to protect patients and health care providers from unintentional radiation exposure. Advise patients to hydrate before and after administration and to void frequently after administration. (2.1, 2.2, 5.2)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥ 1%) were injection site pain, injection site erythema, and injection site irritation (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Life Molecular Imaging at 1‑833-491-2524 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Lactation: Temporarily discontinue breastfeeding. A lactating woman should pump and discard breast milk for 24 hours after NEURACEQ administration (8.2). (7.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2025
Full Prescribing Information
1. Indications and Usage for Neuraceq
NEURACEQ is indicated for positron emission tomography (PET) of the brain to estimate amyloid beta neuritic plaque density in adults with cognitive impairment for:
- Evaluation of Alzheimer’s disease (AD) and other causes of cognitive decline
- Selection of patients who are indicated for amyloid beta-directed therapy as described in the prescribing information of the therapeutic products
2. Neuraceq Dosage and Administration
2.1 Radiation Safety - Drug Handling
Handle NEURACEQ with appropriate safety measures to minimize radiation exposure during administration [see Warnings and Precautions (5.2)].Use waterproof gloves and effective radiation shielding, including lead-glass syringe shields when handling and administering NEURACEQ.
Radiopharmaceuticals, including NEURACEQ, should be used by or under the control of healthcare providers who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
2.2 Recommended Dosing and Administration Instructions
Recommended Dosage
The recommended amount of radioactivity of NEURACEQ is 300 MBq (8.1 mCi) in a total volume of up to 10 mL, administered as a single slow intravenous bolus (6 sec/mL). The maximum mass dose is 30 micrograms. Follow the administration with an intravenous flush of approximately 10 mL of 0.9% sodium chloride injection.
Patient Preparation
Instruct patients to hydrate before and after NEURACEQ administration and to void before imaging and frequently thereafter following NEURACEQ administration [see Warnings and Precautions( 5.2) ].
Administration
- Use aseptic technique and radiation shielding to withdraw and administer NEURACEQ.
- Visually inspect NEURACEQ for particulate matter and discoloration prior to administration. Do not use NEURACEQ if it contains particulate matter or if it is discolored.
- Do not dilute NEURACEQ.
- Measure the activity of NEURACEQ with a dose calibrator immediately prior to injection.
- Verify patency of the indwelling catheter by a test flush with 0.9% sodium chloride injection prior to administration of NEURACEQ.
- Dispose of unused product in a safe manner in compliance with applicable regulations
2.3 Image Acquisition Guideline
- Position the patient supine with the head positioned to center the brain, including the cerebellum, in the PET scanner field of view. Tape or other flexible head restraints may be employed to reduce head movement.
- Acquire 15-minute to 20-minute PET images starting 45 minutes to 130 minutes after NEURACEQ administration.
- Image reconstruction should include attenuation correction with resulting transaxial pixel sizes between 2 mm and 3 mm.
2.4 Image Display and Interpretation
Image Display
- Display images in the transaxial orientation using gray scale or inverse gray scale. The sagittal and coronal planes may be used for additional orientation purposes.
- CT or MR images may be helpful for anatomic reference purposes. However, visual assessment should be performed using the axial planes according to the recommended reading methodology.
- Locate regions which ‘anatomically’ correspond to white matter structures (e.g., the cerebellar white matter or the splenium) for orientation.
- Review images in a systematic manner, starting with the cerebellum and scrolling up through the lateral temporal and frontal lobes, the posterior cingulate cortex/precuneus, and the parietal lobes.
Visual Assessment
NEURACEQ images should be interpreted only by readers who successfully complete training provided by the manufacturer. The reader training can be accessed here: https://www.neuraceqreadertraining.com/learn.
Perform image interpretation independently of the patient’s clinical features, relying on the recognition of unique image features.
Interpret NEURACEQ images based upon the distribution of signal intensity within the cerebral cortex by comparing the signal intensity in the cortical gray matter and the adjacent white matter. Signal intensity in the gray matter is assessed in the following four brain regions: the temporal lobes, the frontal lobes, the posterior cingulate cortex/precuneus, and the parietal lobes. For a gray matter cortical region to be assessed as showing increased signal, the majority of slices from the respective region must be affected. The signal intensity in the cerebellum does not contribute to the scan interpretation. For example, a positive scan may show retained cerebellar gray-white contrast even when the cortical gray-white contrast is lost.
Some scans may be difficult to interpret due to image noise, atrophy with a thinned cortex, or image blur. If co-registered computerized tomography (CT) or magnetic resonance (MR) images are available, the CT/MR images may be used to clarify the relationship of the NEURACEQ uptake and the gray matter anatomy [see Warnings and Precautions (5.1)].
Negative NEURACEQ Scan
Signal intensity in gray matter is lower than in white matter in all four brain regions (no amyloid beta deposition).
A negative scan indicates sparse to no amyloid beta neuritic plaques. In patients being evaluated for AD and other causes of cognitive decline who have not been treated with amyloid beta-directed therapy, a negative scan is inconsistent with a neuropathological diagnosis of AD at the time of image acquisition and reduces the likelihood that a patient’s cognitive impairment is due to AD. A negative scan result does not preclude the accumulation of amyloid beta in the brain in the future.
Positive NEURACEQ Scan
Smaller area(s) of signal intensity equal to or higher than that present in white matter extending beyond the white matter rim to the outer cortical margin involving the majority of the slices within at least one of the four brain regions (“moderate” amyloid beta deposition), or a large confluent area of signal intensity equal to or higher than that present in white matter extending beyond the white matter rim to the outer cortical margin and involving the entire region including the majority of slices within at least one of the four brain regions (“pronounced” amyloid beta deposition). There is no known clinical or histopathologic correlation distinguishing “moderate” from “pronounced” amyloid beta deposition.
A positive scan establishes the presence of moderate to frequent amyloid beta neuritic plaques. Neuropathological examination has shown that moderate to frequent amyloid beta neuritic plaques are present in patients with AD but may also be present in patients with other types of neurologic conditions as well as older people with normal cognition.
Examples of positive and negative scans for each of the four brain regions are illustrated in Figure 1.
Figure 1 Axial view of negative (top row) and positive (bottom row) Neuraceq PET scans
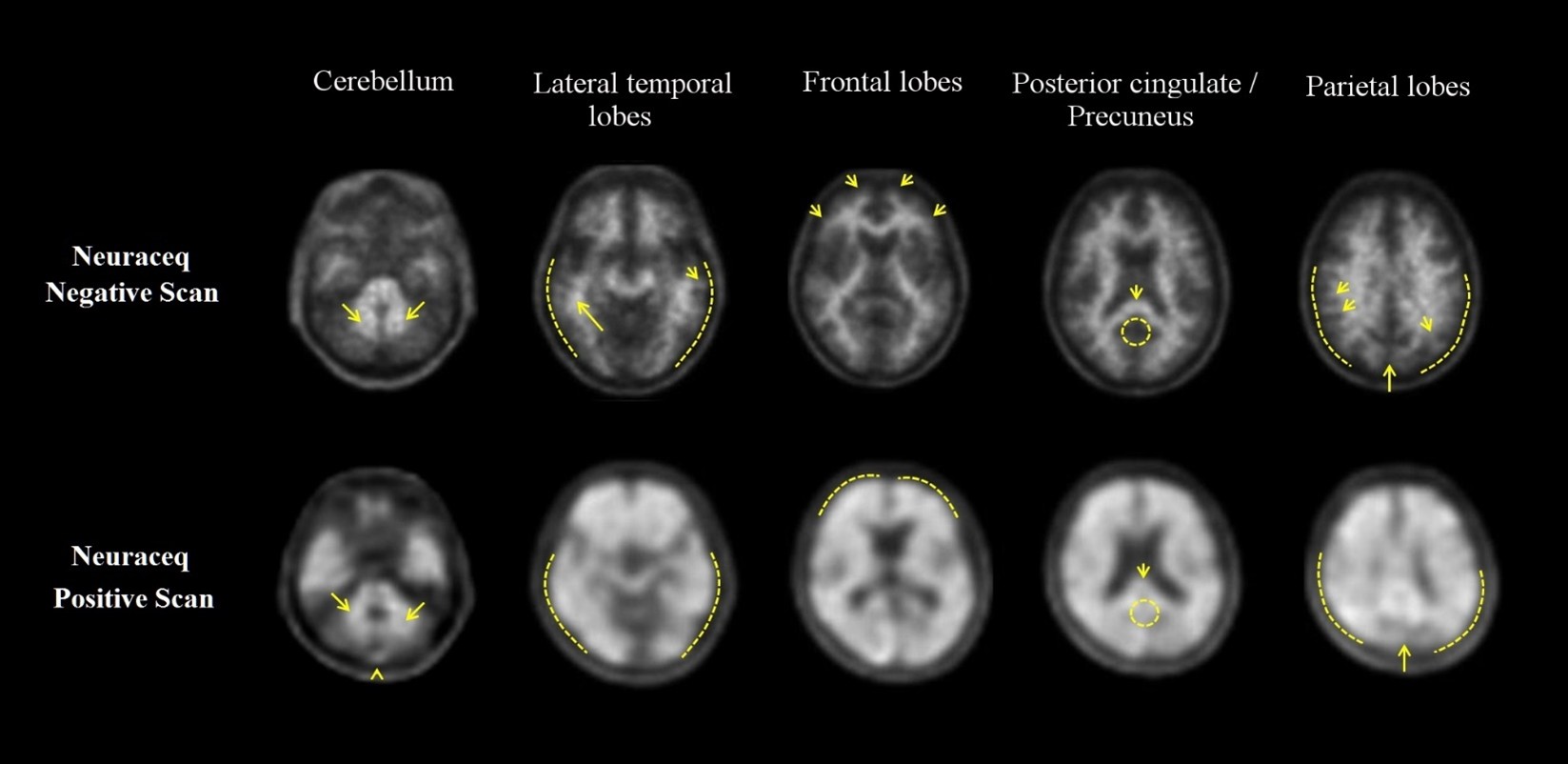
Cerebellum:A contrast between the white matter (arrows) and gray matter is seen in both negative and positive scans. Extracerebral signal intensity in the scalp and posterior sagittal sinus (arrowhead) can be seen. Lateral temporal lobes:Spiculated or “mountainous” appearance of the white matter (arrows) is seen in the negative scan, and the signal does not reach the outer rim of the brain (dashed line) due to lower signal intensity in the gray matter. The positive scan shows a “plumped”, smooth appearance of the outer border of the brain parenchyma (dashed line) due to signal intensity in the gray matter. Frontal Lobes:Spiculated appearance of the white matter in the frontal lobes (arrows) is seen in the negative scan. The positive scan shows “plumped”, smooth appearance in these regions due to the increased gray matter signal intensity (dashed line). Posterior cingulate/precuneus:Regions adjacent and posterior to the splenium (arrow) appear as a hypo-intense “hole” (circle) in the negative scan, whereas this hole is “filled-up” (circle) in the positive scan. Parietal lobes:In the negative scan, the midline between the parietal lobes can be easily identified (long arrow); white matter has a spiculated appearance (short arrow) with low signal near the outer rim of the brain (dashed line). In the positive scan, the midline between the parietal lobes is much thinner. The cortical areas are “filled-up” and are smooth in appearance as signal intensity extends to the outer rim of the brain.
Quantitative Analysis
Quantification of amyloid beta neuritic plaque levels (e.g., Centiloid scale or standardized uptake value ratio (SUVR)) can be used in conjunction with visual assessment and performed with FDA-authorized software indicated for quantification of brain amyloid beta PET scans. Refer to the drug manufacturer’s training materials for qualitative and quantitative assessment and software manufacturers’ documentation for software operation.
2.5 Radiation Dosimetry
Estimated radiation absorbed doses for adults from intravenous injection of Neuraceq are shown in Table 1.
| Organ/Tissue | Mean Absorbed Radiation Dose per Unit Administered Activity
[microGy/MBq] |
| Adrenals | 13 |
| Brain | 13 |
| Breasts | 7 |
| Gallbladder Wall | 137 |
| Heart Wall | 14 |
| Kidneys | 24 |
| Liver | 39 |
| Lower Large Intestine-Wall | 35 |
| Lungs | 15 |
| Muscle | 10 |
| Osteogenic Cells | 15 |
| Ovaries | 16 |
| Pancreas | 14 |
| Red Marrow | 12 |
| Skin | 7 |
| Small Intestine | 31 |
| Spleen | 10 |
| Stomach Wall | 12 |
| Testes | 9 |
| Thymus | 9 |
| Thyroid | 8 |
| Upper Large Intestine-Wall | 38 |
| Urinary Bladder Wall | 70 |
| Uterus | 16 |
| Total Body | 11 |
| Effective Dose (microSv/MBq) | 19 |
The whole-body effective dose resulting from administration of 300 MBq (8.1 mCi) of NEURACEQ in adults is estimated to be 5.8 mSv. When PET/CT is performed, exposure to radiation will increase by an amount dependent on the settings used in the CT acquisition.
3. Dosage Forms and Strengths
Injection: 50 MBq/mL to 5,000 MBq/mL (1.4 mCi/mL to 135 mCi/mL) of florbetaben F 18 in up to 50 mL volume at end of synthesis (EOS) as a clear solution in a multiple-dose vial.
5. Warnings and Precautions
5.1 Risk of Image Misinterpretation and Other Errors
Errors may occur in the estimation of brain amyloid beta neuritic plaque density during NEURACEQ image interpretation [ see Clinical Studies (14)].
The use of clinical information in the interpretation of NEURACEQ images has not been evaluated and may lead to an inaccurate assessment. Severe brain atrophy as well as motion artifacts that result in image distortion may limit the ability to distinguish gray and white matter on a NEURACEQ scan.
Perform image interpretation independently of the patient’s clinical information. For cases where there is uncertainty as to the location of cortical signal, use co-registered anatomical imaging to improve localization of signal [see Dosage and Administration (2.4)].
5.2 Radiation Risk
NEURACEQ contributes to a patient's overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Ensure safe drug handling to protect patients and health care providers from unintentional radiation exposure. Advise patients to hydrate before and after administration and to void frequently after administration [see Dosage and Administration (2.1, 2.2)].
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of NEURACEQ was evaluated in 872 adult subjects who received NEURACEQ by intravenous injection in clinical trials. Of these subjects, 724 received a single dose, 78 received two doses, and 70 received three doses at yearly intervals as part of annual repeat scanning. Table 2 shows adverse reactions reported in 1% of these 1,090 administrations from the clinical trials.
| Adverse Reaction | NEURACEQ
N=1,090 Administrations % |
| Injection site pain | 3.4 |
| Injection site erythema | 1.7 |
| Injection site irritation | 1.1 |
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available data on NEURACEQ use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with florbetaben F 18 to evaluate its effect on female reproduction and embryo-fetal development.
All radiopharmaceuticals, including NEURACEQ, have the potential to cause fetal harm depending on the stage of fetal development and the magnitude of the radiation dose. If considering NEURACEQ administration to a pregnant woman, inform the patient about the potential for adverse pregnancy outcomes based on the radiation dose from the drug and the gestational timing of exposure.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of florbetaben F 18 in human milk, the effects on the breastfed infant, or the effects on milk production. Exposure of NEURACEQ to a breastfed infant can be minimized by temporary discontinuation of breastfeeding (see Clinical Considerations).The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for NEURACEQ and any potential adverse effects on the breastfed child from NEURACEQ or from the underlying maternal condition.
Clinical Considerations
To decrease radiation exposure to the breastfed infant, advise a lactating woman to pump and discard breast milk for 24 hours after administration of NEURACEQ
8.4 Pediatric Use
The safety and effectiveness of NEURACEQ have not been established in pediatric patients.
8.5 Geriatric Use
Of the 872 subjects in clinical studies of NEURACEQ, 603 (69%) subjects were 65 years of age and older, while 304 (35%) subjects were 75 years of age and older. No overall differences in safety or effectiveness were observed between subjects 65 years of age and older and younger adult subjects.
10. Overdosage
In the event of administration of a radiation overdose with NEURACEQ, the absorbed organ dose to the patient should be reduced by increasing elimination of the radionuclide from the body by inducing frequent micturition.
11. Neuraceq Description
11.1 Drug Characteristics
NEURACEQ (florbetaben F 18 injection) is a radioactive diagnostic drug for intravenous use.
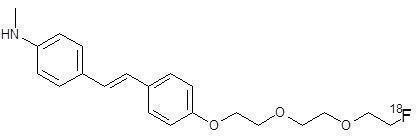
Chemically, florbetaben F 18 is 4-[(E)-2-(4-{2-[2-(2-[ 18F] fluoroethoxy)ethoxy]ethoxy}phenyl)vinyl]-N-methylaniline. The molecular weight is 358.45 and the structural formula is:
NEURACEQ is a sterile, non-pyrogenic, clear solution. Each mL contains up to 3 micrograms of florbetaben and 50 MBq to 5,000 MBq (1.4 mCi to 135 mCi) of florbetaben F 18 at EOS with the following inactive ingredients: 4.4 mg ascorbic acid, 118 mg ethanol, 200 mg macrogol 400, and 28.8 mg sodium ascorbate. The pH of the solution is between 4.5 and 7.
11.2 Nuclear Physical Characteristics
Fluorine-18 (F 18) decays by positron (ß +) emission to oxygen-18 and has a physical half-life of 109.8 minutes. The principal photons useful for diagnostic imaging are the coincident pair of 511 keV gamma photons, resulting from the interaction of the emitted positron with an electron (Table 3).
|
Radiation |
Energy Level (keV) |
Abundance (%) |
|
Positron |
249.8 |
96.7 |
|
Gamma |
511 |
193.4 |
The point source air-kerma coefficient for F 18 is 3.74E -17 Gy m 2/(Bq s); this coefficient was formerly defined as the specific gamma-ray constant of 5.7 R/hr/mCi at 1 cm. The first half-value thickness of lead for F 18 gamma rays is approximately 6 mm. The relative reduction of radiation emitted by F 18 that results from various thicknesses of lead shielding is shown in Table 4. The use of ~8 cm of lead will decrease the radiation transmission (i.e., exposure) by a factor of about 10,000.
|
Shield Thickness
|
Coefficient of Attenuation |
|
0.6 |
0.5 |
|
2 |
0.1 |
|
4 |
0.01 |
|
6 |
0.001 |
|
8 |
0.0001 |
For use in correcting for physical decay of this radionuclide, the fractions remaining at selected intervals after calibration are shown in Table 5.
| Minutes | Fraction Remaining |
| 0 | 1.00 |
| 15 | 0.910 |
| 30 | 0.828 |
| 60 | 0.685 |
| 110 | 0.500 |
| 220 | 0.250 |
| 440 | 0.063 |
12. Neuraceq - Clinical Pharmacology
12.1 Mechanism of Action
Florbetaben F 18 binds to amyloid beta plaques in the brain and the F 18 isotope produces a positron signal that is detected by a PET scanner. 3H-florbetaben in vitro binding experiments revealed two binding sites (K dof 16 nM and 135 nM) in frontal cortex homogenates from patients with AD. Binding of florbetaben F 18 to amyloid beta plaques in postmortem brain sections from patients with AD using autoradiography correlated with both immunohistochemical and Bielschowsky silver stains. Florbetaben F 18 did not bind to tau or α-synuclein in tissue from patients with AD. Neither florbetaben F 18 nor non-radioactive florbetaben F 19 bound to AT8 positive tau deposited in brain tissue from patients with frontotemporal dementia (FTD), using autoradiography and immunohistochemistry, respectively.
12.2 Pharmacodynamics
Following intravenous administration, florbetaben F 18 crosses the human blood-brain barrier and shows differential retention in brain regions that contain amyloid beta deposits. Differences in signal intensity between brain regions showing specific and non-specific florbetaben F 18 uptake form the basis for the image interpretation method [see Dosage and Administration (2.4)].
12.3 Pharmacokinetics
Following intravenous administration of 300 MBq (8.1 mCi) of NEURACEQ in healthy subjects, approximately 6% of the injected radioactivity was distributed to the brain at 10 minutes post-injection. Florbetaben F 18 plasma concentrations declined by approximately 75% at 20 minutes post-injection, and by approximately 90% at 50 minutes. The F 18 in circulation during the 45-minute to 130-minute imaging window was principally in the form of polar metabolites of florbetaben. Florbetaben F 18 was 98.5% bound to plasma proteins and was eliminated from plasma primarily via the hepatobiliary route with a mean biological half-life of approximately 1 hour.
In vitrostudies show that metabolism of florbetaben is predominantly catalyzed by CYP2J2 and CYP4F2. At 12 hours post-administration, approximately 30% of the injected radioactivity had been excreted in urine. Almost all F 18 radioactivity in urine was excreted as polar metabolites of florbetaben F 18 and only trace amounts of florbetaben F 18 were detected.
In in vitrostudies using human liver microsomes, florbetaben did not inhibit cytochrome P450 enzymes at concentrations present in vivo.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not been performed to evaluate the potential for carcinogenesis, impairment of male or female fertility, or reproductive toxicity by florbetaben F 18.
Mutagenesis
Florbetaben did not demonstrate mutagenic potential in an in vitrobacterial mutation assay (Ames test) using five strains of Salmonella typhimuriumand one strain of Escherichia colior in an in vitrochromosomal aberration assay using human peripheral lymphocytes in the absence and presence of a metabolic activator.
14. Clinical Studies
14.1 Evaluation of AD and Other Causes of Cognitive Decline
The effectiveness of NEURACEQ was evaluated in two single-arm clinical studies (i.e., Studies 1 and 2) in subjects with a range of cognitive function, including some end-of-life subjects who had agreed to participate in a postmortem brain donation program as well as healthy subjects. Subjects underwent a NEURACEQ injection and scan. Images were interpreted using a clinically applicable binary image interpretation method (negative or positive) by independent readers blinded to all clinical information [see Dosage and Administration (2.4)]. Before image interpretation, all readers underwent either in-person or electronic media training on image interpretation.
The truth standard was based on the histopathologic examination using Bielschowsky silver staining (BSS) of six brain regions assessed by a pathology consensus panel blinded to all clinical information (including PET scan results). NEURACEQ results (negative or positive) were pre-specified to correspond with specific histopathology-derived plaque density scores, based upon a modification of the Consortium to Establish a Registry for Alzheimers Disease (CERAD) criteria, which use neuritic plaque counts as a necessary pathological feature of AD (Table 6). For the subject-level truth standard, if any of the six regions were scored as more than sparse, the subject was classified as positive; if none of the regions were assessed as being more than sparse, the subject was classified as negative.
|
Histopathology Categorization |
NEURACEQ PET Read |
|
|
Neuritic Plaque Counts |
CERAD Score |
|
|
<1 |
None |
Negative |
|
1-5 |
Sparse |
|
|
6-19 |
Moderate |
Positive |
|
>20 |
Frequent |
|
Study 1 evaluated performance characteristics (sensitivity and specificity) in subjects who died during the study by comparing premortem NEURACEQ PET images to a postmortem truth standard of amyloid beta neuritic plaque density. A total of 205 subjects were dosed with NEURACEQ and imaged. The mean age was 77 years (range 48 to 98 years) and 52% of the subjects were male. By medical history, 137 subjects had AD, 31 had other non-AD dementia, 5 had dementia with Lewy bodies (DLB), and 32 had no clinical evidence of dementia. Among the imaged subjects, 82 subjects died during the study and were included in the primary analysis. The time interval between the NEURACEQ scan and death was less than one year for 45 subjects, between one and two years for 23 subjects, and more than two years for 14 subjects. At autopsy, the subject-level brain amyloid beta neuritic plaque density category was frequent (n = 31), moderate (n = 21), sparse (n = 17), or none (n = 13). Three readers, after undergoing in-person training, interpreted images from the 82 autopsied subjects. Five independent, blinded readers underwent electronic media training and assessed images from the same 82 end-of-life subjects.
Study 2 evaluated inter-reader and intra-reader reproducibility of image interpretation using images from subjects with a truth standard (60 subjects who participated in Study 1) and subjects without a truth standard (50 subjects with mild cognitive impairment (MCI), 139 subjects with AD, 30 subjects with other non-AD dementias/disease, 5 subjects with Parkinsons disease (PD), and 170 healthy subjects). Intra-reader reproducibility was assessed with images from 46 subjects (10%). Among the 454 subjects, the mean age was 71 years (range 22 to 98 years), 193 were females, and 353 were White. Readers underwent electronic media training.
Among subjects who underwent autopsy (n=82; 52 positive and 30 negative based on histopathology), the sensitivity using the majority interpretation of the in-person trained readers (three readers) was 96.2% (95% CI: 86.8%, 99.5%) and specificity was 80.0% (95% CI: 61.4% to 92.3%). The Sensitivity of the readers trained using electronic media (five readers) was 96.2% (95% CI: 86.8%, 99.5%) and specificity was 76.7% (95% CI: 57.7% to 90.1%). The median (and range) of correct, false negative, and false positive reads were 71 (65 to 73), 2 (0 to 5), 7 (6 to 16), respectively, for electronic media training; and were 75 (74, 75), 1 (1, 2), 6 (5, 7), respectively, for in-person training.
Table 7 shows the inter-reader reproducibility for various subject groups in Study 2 a. Inter-reader agreement analyses across all five readers showed an overall Fleiss kappa statistic of 0.79 (95% CI: 0.77, 0.83), which met the pre-specified success criterion (95% CI lower bound > 0.60). The percentage of intra-reader agreement for the five readers ranged from 91% to 98%.
|
Subject Group by Cognitive Status and Truth Standard (TS) |
Positive Scans, n b |
Kappa (95% CI) |
Percent of Scans with Inter-Reader Agreement |
||
|
3 of 5 readers agreed |
4 of 5 readers agreed |
5 of 5 readers agreed |
|||
|
All subjects, n=454 |
212 |
0.80 (0.77, 0.83) |
6 |
15 |
78 |
|
Subjects with TS, n=60 |
37 |
0.75 (0.67, 0.83) |
10 |
15 |
75 |
|
Subjects without TS, n=394 |
175 |
0.80 (0.77, 0.83) |
6 |
15 |
79 |
|
AD, n=176 (37 with TS; 139 no TS) |
139 |
0.77 (0.72, 0.81) |
7 |
10 |
83 |
|
MCI without TS, n=50 |
28 |
0.84 (0.75, 0.92) |
0 |
20 |
80 |
|
Other non-AD dementia c, n=40 (5 with TS; 35 no TS) |
18 |
0.65 (0.55, 0.74) |
8 |
33 |
60 |
|
Healthy subjects, n=188 (18 with TS; 170 no TS) |
26 |
0.55 (0.49, 0.58) |
7 |
15 |
77 |
aReaders who underwent electronic media training.
bShown is the median number of scans interpreted as positive across the five readers for each group of subjects listed in the first column.
cOther non-AD dementia includes dementia with Lewy bodies (DLB), fronto-temporal lobe dementia, vascular dementia, and dementia associated with Parkinson’s disease (PD).
14.2 Selection of Patients Indicated for Amyloid Beta-Directed Therapy
Refer to the prescribing information of amyloid beta-directed therapy for description of clinical trials in which the efficacy of amyloid beta PET for selecting patients has been established.
Brain amyloid beta PET scans have been used to assess reduction of plaque in some clinical trials of amyloid beta-directed therapies as also described in the prescribing information of the therapeutic products.
16. How is Neuraceq supplied
How Supplied
NEURACEQ (florbetaben F 18 injection) is a clear solution supplied at a concentration of 50 MBq/mL to 5,000 MBq/mL (1.4 mCi/mL to 135 mCi/mL) of florbetaben F 18 in up to 50 mL volume at EOS in a shielded multiple-dose glass vial (NDC 54828-001-50).
Storage and Handling
Store NEURACEQ in the original container with radiation shielding at room temperature 25°C (77°F); excursions permitted to 2°C to 42°C (36°F to 108°F).
NEURACEQ does not contain a preservative.
Do not use after the expiration date and time provided on the container label.
NEURACEQ multiple-dose vial expires 10 hours after EOS.
Dispose of unused product in accordance with all federal, state, and local laws and institutional requirements.
This preparation is for use by persons under license by the Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State.
17. Patient Counseling Information
Radiation Risk
Advise patients of the radiation risk of NEURACEQ. Instruct patients to increase their level of hydration before and after receiving NEURACEQ and to void frequently following administration [see Warnings and Precautions (5.2)].
Pregnancy
Inform pregnant women of the potential risks of fetal exposure to radiation doses with NEURACEQ [see Use in Specific Populations (8.1)].
Lactation
Advise a lactating woman to temporarily discontinue breastfeeding and to pump and discard breast milk for 24 hours after NEURACEQ administration to minimize radiation exposure to the breastfed infant [see Use in Specific Populations (8.2)].
| NEURACEQ
florbetaben f 18 injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Life Molecular Imaging, Ltd (735628575) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SOFIE Co. dba SOFIE | 829109441 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SOFIE Co. dba SOFIE | 079854636 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SOFIE Co. dba SOFIE | 928455851 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SOFIE Co. dba SOFIE | 025600556 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SOFIE Co. dba SOFIE | 032324142 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SOFIE Co. dba SOFIE | 832599976 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SOFIE Co. dba SOFIE | 006320902 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SOFIE Co. dba SOFIE | 078623800 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SOFIE Co. dba SOFIE | 118258354 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SOFIE Co. dba SOFIE | 008017970 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| N-Molecular, Inc. dba SOFIE | 079932634 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| N-Molecular, Inc. dba SOFIE | 079932640 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| N-Molecular, Inc. dba SOFIE | 079932600 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Jubilant DraxImage Inc. dba Jubilant Radiopharma | 080871737 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Jubilant DraxImage Inc. dba Jubilant Radiopharma | 080871701 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PharmaLogic Colorado, LLC | 117608150 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PharmaLogic Cincinnati, LLC | 118408248 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PharmaLogic Salt Lake City, LLC | 119319723 | positron emission tomography drug production(54828-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PharmaLogic Los Angeles, LLC | 119024924 | positron emission tomography drug production(54828-001) | |
More about Neuraceq (florbetaben f-18)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: diagnostic radiopharmaceuticals
- Breastfeeding