Nephroscan: Package Insert / Prescribing Info
Package insert / product label
Generic name: succimer
Dosage form: injection, powder, lyophilized, for solution
Medically reviewed by Drugs.com. Last updated on Oct 6, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
NEPHROSCAN (kit for the preparation of technetium Tc 99m succimer injection), for intravenous use
Initial U.S. Approval: 1982
Indications and Usage for Nephroscan
NEPHROSCAN, after radiolabeling with technetium Tc 99m, is a radioactive diagnostic agent indicated for use as an aid in the scintigraphic evaluation of renal parenchymal disorders in adults and pediatric patients including term neonates. (1)
Nephroscan Dosage and Administration
- Instruct patients to drink a sufficient amount of water before administration and to continue to drink and to void frequently following administration (2.2)
- The recommended amount of radioactivity by intravenous injection (bolus) is:
- Begin image acquisition 1 hour to 4 hours after administration (2.7)
- Delay imaging up to 6 hours to 24 hours in patients with severely reduced eGFR (2.7)
- See Full Prescribing Information for radiation safety, drug preparation, administration, and radiation dosimetry information (2.1, 2.4, 2.5, 2.6, 2.8)
Dosage Forms and Strengths
For Injection: NEPHROSCAN contains 1 mg succimer as a white-yellowish to off-white lyophilized powder in a single dose vial. Upon radiolabeling with technetium Tc 99m, it provides a clear, colorless solution containing up to 1,480 MBq (40 mCi) technetium Tc 99m succimer in approximately 5 mL volume at calibration time. (3)
Contraindications
None (4)
Warnings and Precautions
- Hypersensitivity Reactions: Have appropriate instruments and medications necessary for immediate treatment of allergic reactions and monitor patients for hypersensitivity reactions during and after administration (5.1)
- Radiation risks: Ensure safe handling to minimize radiation exposure to patients and health care workers (5.2)
- Risk in Patients with Advanced Renal Failure: Image interpretation may be affected as the kidneys may not absorb technetium Tc 99m succimer. (5.3)
Adverse Reactions/Side Effects
The following adverse reactions have been reported: urticaria, rash, pruritus, erythema, syncope, fever, and nausea. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Theragnostics, Inc., at 1-888-286-3848 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Lactation: Temporarily discontinue breastfeeding. A lactating woman should pump and discard breastmilk for at least 24 hours after Technetium Tc 99m Succimer Injection administration. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2024
Full Prescribing Information
1. Indications and Usage for Nephroscan
NEPHROSCAN, after radiolabeling with technetium Tc 99m, is indicated for use as an aid in the scintigraphic evaluation of renal parenchymal disorders in adult and pediatric patients including term neonates.
2. Nephroscan Dosage and Administration
2.1 Radiation Safety – Drug Handling
After radiolabeling, handle Technetium Tc 99m Succimer Injection with appropriate safety measures to minimize radiation exposure [see Warnings and Precautions (5.2)]. Use waterproof gloves, effective radiation shielding, and other appropriate safety measures when preparing and handling Technetium Tc 99m Succimer Injection.
Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
2.2 Patient Preparation
Instruct patients to drink a sufficient amount of water to ensure adequate hydration prior to administration of Technetium Tc 99m Succimer Injection and to continue to drink and void frequently following administration to reduce radiation exposure [see Warnings and Precautions (5.2)].
2.3 Recommended Dosage
Adults
The recommended amount of radioactivity of Technetium Tc 99m Succimer Injection for renal parenchymal imaging in adults is 74 MBq to 222 MBq (2 mCi to 6 mCi) by intravenous injection (bolus).
Pediatric Patients
The recommended amount of radioactivity of Technetium Tc 99m Succimer Injection for renal parenchymal imaging in pediatric patients is 1.85 MBq/kg (0.05 mCi/kg) of body weight with a range of 19 MBq to 74 MBq (0.5 mCi to 2 mCi) by intravenous injection (bolus). Weight based pediatric dosing is shown in Table 1.
| Body Weight (kg) | Recommended Radioactivity MBq (mCi) | Body Weight (kg) | Recommended Radioactivity MBq (mCi) |
|---|---|---|---|
| less than 11 kg | 19 MBq (0.5 mCi) | 25 to 26 | 49 MBq (1.3 mCi) |
| 11 to 12 | 21 MBq (0.6 mCi) | 27 to 28 | 52 MBq (1.4 mCi) |
| 13 to 14 | 26 MBq (0.7 mCi) | 29 to 30 | 56 MBq (1.5 mCi) |
| 15 to 16 | 30 MBq (0.8 mCi) | 31 to 32 | 59 MBq (1.6 mCi) |
| 17 to 18 | 33 MBq (0.9 mCi) | 33 to 34 | 63 MBq (1.7 mCi) |
| 19 to 20 | 37 MBq (1 mCi) | 35 to 36 | 67 MBq (1.8 mCi) |
| 21 to 22 | 41 MBq (1.1 mCi) | 37 to 38 | 70 MBq (1.9 mCi) |
| 23 to 24 | 44 MBq (1.2 mCi) | 39 or greater | 74 MBq (2 mCi) |
2.4 Drug Preparation
Prepare Technetium Tc 99m Succimer Injection according to the following procedure using asceptic technique:
- Wear waterproof gloves.
- Place the kit vial in lead shielding and disinfect the stopper (allow disinfectant to dry).
- Use a sterile syringe to transfer 5 mL sodium pertechnetate Tc 99m injection obtained from a technetium Tc 99m generator with a maximum activity of 1,480 MBq (40 mCi) to the vial. The volume of sodium pertechnetate Tc 99m injection may be adjusted to 5 mL prior to adding to the kit vial using 0.9% sodium chloride injection, USP.
- Use the same syringe to withdraw the appropriate gas volume from the vial for pressure compensation.
- Lightly shake the vial to completely dissolve the powder. Ensure the stopper is well moistened.
- Incubate the vial for 10 minutes at controlled room temperature 20°C to 25°C (68°F to 77°F).
- Measure the product activity in a dose calibrator; complete the radiolabeled product vial label and affix to the vial shield.
- Check radiochemical purity according to the method in 2.5 Radiochemical Purity Determination [see Dosage and Administration (2.5)].
- Use Technetium Tc 99m Succimer Injection within 4 hours and store at controlled room temperature 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
2.5 Radiochemical Purity Determination
Determine the radiochemical purity of Technetium Tc 99m Succimer Injection as follows:
- Activate a 65 × 95-mm silicic acid thin-layer chromatographic (TLC) plate by heating at 100°C to 110°C (212°F to 230°F) for 30 minutes.
- Cool the TLC plate over silica gel and immediately apply 1 microL of Technetium Tc 99m Succimer Injection about 17 mm from one end of the TLC plate and allow to dry. If necessary, the Technetium Tc 99m Succimer Injection may be diluted with 0.9% sodium chloride injection USP, to a radioactive concentration of 18.5 MBq to 370 MBq (0.5 mCi to 10 mCi) per mL.
- Develop the TLC plate over a period of about 30 minutes to 45 minutes by ascending chromatography, using a solution of n-butanol saturated with 0.3 N hydrochloric acid (see Note 1). Air-dry the developed TLC Plate.
- Determine the radioactive distribution on the TLC plate by scanning the chromatogram with a radiochromatographic scanner having a suitably collimated radiation detector.
- The radioactivity associated with technetium Tc 99m succimer is at Rf between 0.45 and 0.7, hydrolyzed Tc 99m is located at the origin (Rf 0 to 0.15) and the unbound Tc 99m is located at the solvent front (Rf 1)
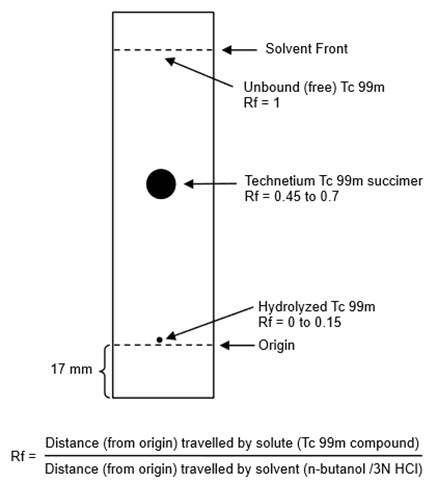
- Calculate radiochemical purity using the following formula:

- Technetium Tc 99m Succimer Injection preparation with not less than 85% radiochemical purity is suitable for administration. Discard preparation with less than 85% radiochemical purity.
Note 1: To prepare the n-butanol saturated with 0.3 N hydrochloric acid solution, place 50 mL of 0.3 N HCl and 50 mL of n-butanol in an Erlenmeyer flask. Place the mixture in an ultrasonic bath for 2 hours, during which time the solution heats up to about 50°C. Cool the solution to room temperature. After about 30 minutes to 45 minutes the phase separation of the mixture will complete. Collect the upper phase of the mixture and discard the lower phase. The solution is stable for up to 7 days when stored at controlled room temperature 20°C to 25°C (68°F to 77°F).
2.6 Administration
Prior to use, visually inspect the prepared Technetium Tc 99m Succimer Injection behind a lead glass shield. Only use solutions that are clear without visible particles.
In making dosage calculations, correction is to be made for radioactive decay. The radioactive half-life of Tc 99m is 6.0 hours.
Using a sterile shielded syringe, aseptically withdraw the prepared Technetium Tc 99m Succimer Injection, and measure the radioactivity in the syringe using a dose calibrator, prior to administration. Ensure that the injected radioactivity is within ±10% of the recommended dose.
Discard unused portion. Handle and dispose radioactive material in accordance with applicable regulations.
2.7 Image Acquisition
The patient should be placed in the prone or supine position, as required by scanning equipment characteristics. Begin image acquisition 1 hour to 4 hours after the intravenous administration of Technetium Tc 99m Succimer Injection.
Delay image acquisition up to 6 hours to 24 hours in patients with severely reduced glomerular filtration rate (eGFR). A specific eGFR threshold at which to delay imaging has not been established [see Warnings and Precautions (5.3)].
2.8 Radiation Dosimetry
The estimated absorbed radiation doses to an average adult and pediatric patients are shown in Table 2.
| Absorbed Dose per Unit of Activity Administered (mGy/MBq) | |||||
|---|---|---|---|---|---|
| Organ | Adults | 15 years | 10 years | 5 years | 1 year |
| Adrenals | 0.012 | 0.016 | 0.024 | 0.035 | 0.06 |
| Bladder wall | 0.018 | 0.023 | 0.029 | 0.031 | 0.057 |
| Bone surface | 0.005 | 0.0062 | 0.0092 | 0.014 | 0.026 |
| Brain | 0.0012 | 0.0015 | 0.0025 | 0.004 | 0.0072 |
| Breasts | 0.0013 | 0.0018 | 0.0028 | 0.0045 | 0.0084 |
| Gall bladder | 0.0083 | 0.01 | 0.014 | 0.022 | 0.031 |
| Stomach wall | 0.0052 | 0.0063 | 0.01 | 0.014 | 0.02 |
| Colon | 0.005 | 0.0063 | 0.01 | 0.014 | 0.024 |
| Intestine | 0.0043 | 0.0055 | 0.0082 | 0.012 | 0.02 |
| Upper large intestine | 0.005 | 0.0064 | 0.095 | 0.014 | 0.023 |
| Lower large intestine | 0.0035 | 0.0043 | 0.0065 | 0.0096 | 0.016 |
| Heart | 0.003 | 0.0038 | 0.0058 | 0.0086 | 0.014 |
| Kidneys | 0.18 | 0.22 | 0.3 | 0.43 | 0.76 |
| Liver | 0.0095 | 0.012 | 0.018 | 0.025 | 0.041 |
| Lungs | 0.0025 | 0.0035 | 0.0052 | 0.008 | 0.015 |
| Muscles | 0.0029 | 0.0036 | 0.0052 | 0.0077 | 0.014 |
| Oesophagus | 0.0017 | 0.0023 | 0.0034 | 0.0054 | 0.0094 |
| Ovaries | 0.0035 | 0.0047 | 0.007 | 0.011 | 0.019 |
| Pancreas | 0.009 | 0.011 | 0.016 | 0.023 | 0.037 |
| Red marrow | 0.0039 | 0.0047 | 0.0068 | 0.009 | 0.014 |
| Skin | 0.0015 | 0.0018 | 0.0029 | 0.0045 | 0.0085 |
| Spleen | 0.013 | 0.017 | 0.026 | 0.038 | 0.061 |
| Testes | 0.0018 | 0.0024 | 0.0037 | 0.0053 | 0.01 |
| Thymus | 0.0017 | 0.0023 | 0.0034 | 0.0054 | 0.0094 |
| Thyroid | 0.0015 | 0.0019 | 0.0031 | 0.0052 | 0.0094 |
| Uterus | 0.0045 | 0.0056 | 0.0083 | 0.011 | 0.019 |
| Remaining organ | 0.0029 | 0.0037 | 0.0052 | 0.0077 | 0.014 |
| Effective Dose per unit of activity administered (mSv/MBq) | 0.0088 | 0.011 | 0.015 | 0.021 | 0.037 |
3. Dosage Forms and Strengths
For Injection: NEPHROSCAN contains 1 mg succimer as a white-yellowish to off-white lyophilized powder in a single-dose vial. Upon radiolabeling with technetium Tc 99m, it provides up to 1,480 MBq (40 mCi) Technetium Tc 99m Succimer Injection as a clear, colorless solution in approximately 5 mL volume at calibration date and time.
5. Warnings and Precautions
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including urticaria, rash, pruritus, and erythema have been reported with the use of technetium Tc 99m succimer injection in adults and pediatric patients. The time of onset of the reactions varied within 2 hours to several hours after the injection. Have appropriate instruments and medications necessary for immediate treatment of hypersensitivity reactions and monitor patients for reactions during and after administration [see Adverse Reactions (6)].
5.2 Radiation Risks
Technetium Tc 99m succimer injection contributes to a patient's overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk for cancer. Ensure safe handling to minimize radiation exposure to the patient and health care workers. Advise patients to hydrate before and after administration and to void frequently after administration [see Dosage and Administration (2.1, 2.2)].
5.3 Risk in Patients with Advanced Renal Failure
The use of technetium Tc 99m succimer injection in patients with severely reduced estimated glomerular filtration (eGFR) may have an effect on image interpretation as the kidneys may not absorb the technetium Tc 99m succimer and thus the Tc 99m succimer may distribute to organs or parts of the body other than the kidneys. It has been reported that satisfactory images may be obtained in some of these patients by delaying imaging between 6 hours to 24 hours [see Dosage and Administration (2.7)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are discussed elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
The following adverse reactions associated with the use of NEPHROSCAN were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Hypersensitivity including urticaria, rash, pruritus, and erythema
General Disorders: syncope, fever, and nausea.
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Available data with technetium Tc 99m succimer use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects and miscarriage. Animal reproduction studies with technetium Tc 99m succimer have not been conducted. Although all radiopharmaceuticals have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose, the radiation exposure to the fetus from technetium Tc 99m succimer is expected to be low (less than 0.50 mGy) (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies are 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
Technetium Tc 99m succimer is present in breast milk. There are no data on the effects of technetium Tc 99m succimer on the breastfed infant or the effects on milk production. NEPHROSCAN is used for imaging in infants with renal disease; exposure to technetium Tc 99m succimer via breast milk is expected to be lower. Based on clinical guidelines, exposure of technetium Tc 99m succimer to a breastfed infant may be minimized by advising a lactating woman to temporarily discontinue breastfeeding and to pump and discard breast milk for a minimum of at least 24 hours after administration of Technetium Tc 99m Succimer Injection. The developmental and health benefits of breastfeeding should be considered along with a mother's clinical need for NEPHROSCAN, any potential adverse effects on the breastfed child from technetium Tc 99m succimer or from the underlying maternal condition.
8.4 Pediatric Use
NEPHROSCAN, after radiolabeling with technetium Tc 99m, is indicated for use as an aid in the scintigraphic evaluation of renal parenchymal disorders in pediatric patients, including term neonates. Use of NEPHROSCAN in this age group for this indication is supported by evidence from effectiveness established in adult studies and data from published pediatric studies supporting the safety and effectiveness of weight-based dosing of Technetium Tc 99m Succimer Injection in renal parenchymal imaging in pediatric patients including term neonates [see Dosage and Administration (2.3)].
The recommended amount of radioactivity in pediatric patients, 1.85 MBq/kg (0.05 mCi/kg) with a range of 19 MBq to 74 MBq (0.5 mCi to 2 mCi), is based on published studies that used technetium Tc 99m succimer for the evaluation of acute pyelonephritis, renal scarring, and split renal function in pediatric patients [see Dosage and Administration (2.2)].
Hypersensitivity reactions, including urticaria, rash, pruritus, and erythema have been reported with the use of technetium Tc 99m succimer in pediatric patients [see Warnings and Precautions (5.1) and Adverse Reactions (6)].
8.5 Geriatric Use
Clinical studies of technetium Tc 99m succimer did not include sufficient numbers of subjects 65 years of age and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious usually administering the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with decreased renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
10. Overdosage
In the event of an overdose of technetium Tc 99m succimer, reduce the radiation absorbed dose to the patient where possible by increasing the elimination of the drug from the body using hydration and frequent bladder voiding. A diuretic might also be considered. If possible, an estimate of the radiation effective dose given to the patient should be made.
11. Nephroscan Description
11.1 Chemical Characteristics
NEPHROSCAN is a sterile, single-dose kit for the preparation of Technetium Tc 99m Succimer Injection a radioactive diagnostic agent, for intravenous use.
The active ingredient, succimer is meso-2,3-dimercaptosuccinic acid with a molecular weight of 182.22 g/mol. The chemical structure of succimer is shown below. Subsequent to radiolabeling with sodium pertechnetate Tc 99m, the active moiety technetium Tc 99m succimer is obtained in situ.
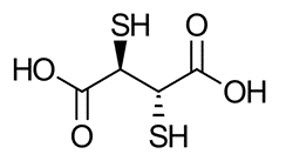
Each vial contains 1 mg succimer, 0.1 mg ascorbic acid, 0.42 mg stannous (tin) chloride dihydrate, 0.02 mg hydrochloric acid and 0.2 mg sodium hydroxide as a white-yellowish to off-white lyophilized powder.
In addition, after radiolabeling with sodium pertechnetate Tc 99m injection, each vial contains up to 1,480 MBq (40 mCi) of technetium Tc 99m succimer in 0.9% sodium chloride injection as a sterile, clear, and colorless solution. pH of the final solution is between 3 and 3.5. FDA approved pH specifications differ from USP.
11.2 Physical Characteristics
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours. The principal photon that is useful for detection and imaging studies is listed in Table 3.
| Radiation/ Emission | Mean % / Disintegration | Mean Energy (keV) |
|---|---|---|
| Gamma 2 | 89.07 | 140.5 |
To correct for physical decay of this radionuclide, the fractions that remain at selected intervals after the time of calibration are shown in Table 4.
11.3 External Radiation
The specific gamma ray constant for technetium Tc 99m is 0.78 R/hr-mCi at 1 cm. The first half value layer is 0.017 cm of Pb. To facilitate control of the radiation exposure from millicurie amounts of this radionuclide, the use of a 0.25 cm thickness of Pb will attenuate the radiation emitted by a factor of about 1,000. Table 5 displays the radiation attenuation by lead shielding.
| Shield Thickness (Pb) mm | Coefficient of Attenuation |
|---|---|
| 0.02 | 0.5 |
| 0.08 | 0.1 |
| 0.16 | 0.01 |
| 0.25 | 0.001 |
| 0.33 | 0.0001 |
12. Nephroscan - Clinical Pharmacology
12.1 Mechanism of Action
Technetium Tc 99m succimer binds to the cortical region of kidneys and in conjunction with gamma scintigraphy or single photon emission computed tomography (SPECT) is used to image the renal cortices.
12.2 Pharmacodynamics
Technetium Tc 99m succimer only stays bound to viable cortical tissue for >24 hours following administration. The relationship between technetium Tc 99m succimer plasma concentrations and successful imaging is not known.
12.3 Pharmacokinetics
Distribution
Technetium Tc 99m succimer is distributed in the plasma, bound to plasma proteins following intravenous administration. In humans, 53% to 70% of the intravenously administered technetium Tc 99m is protein bound. There is negligible activity in the red blood cells. At 6 hours about 20% of the dose is concentrated in each kidney.
16. How is Nephroscan supplied
How Supplied
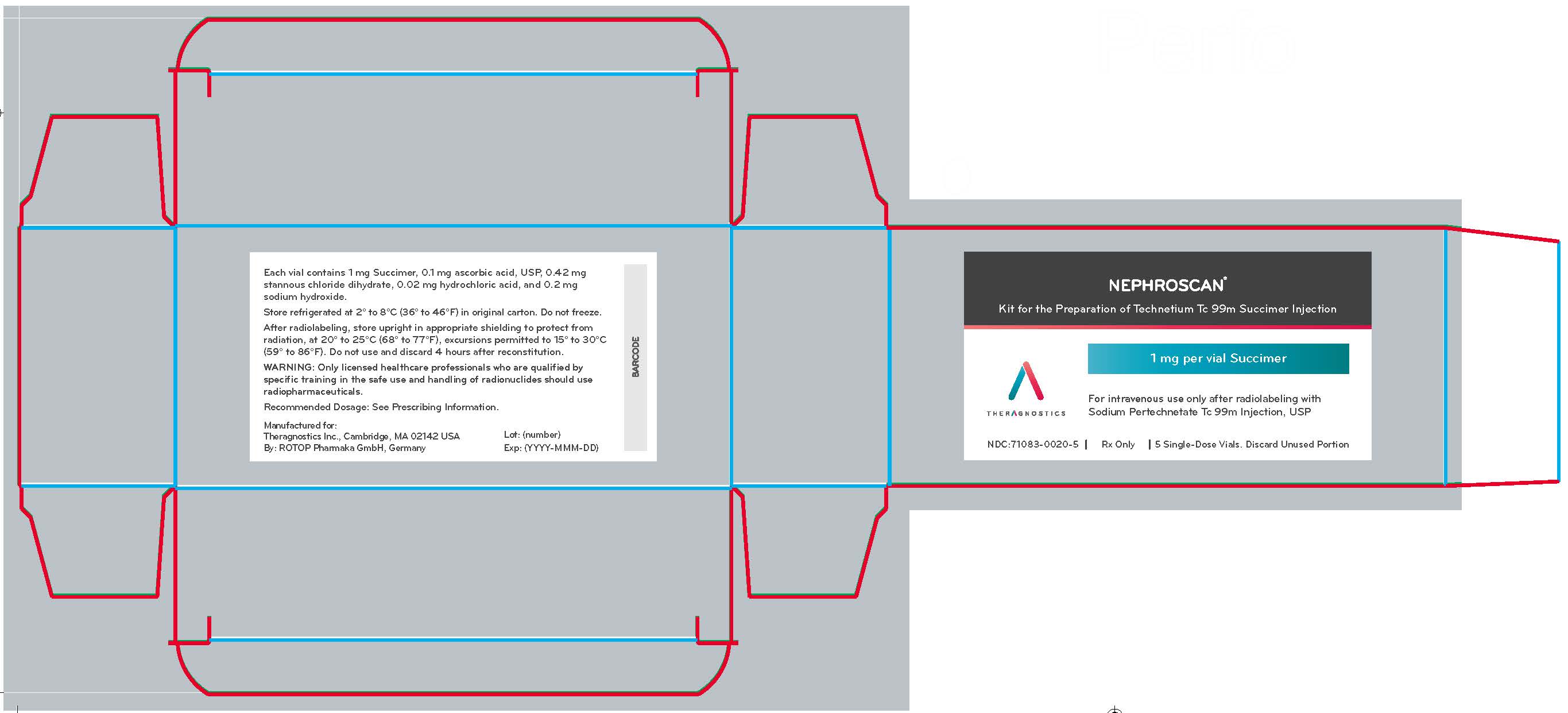
NEPHROSCAN (kit for the preparation of technetium Tc 99m succimer injection), containing 1 mg succimer, is supplied as a white-yellowish to off-white lyophilized powder packaged in a sterile, single-dose vial as a carton of 5 vials (NDC 71083-0020-5).
Storage and Handling
Before radiolabeling, store NEPHROSCAN in a refrigerator at 2°C to 8°C (36°F to 46°F) in original carton. Do not freeze.
After radiolabeling, store Technetium Tc 99m Succimer Injection upright, shielded, for up to 4 hours, at controlled room temperature 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see Dosage and Administration (2.4)].
This preparation is approved for use by persons under license by the Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State.
17. Patient Counseling Information
Adequate Hydration
Instruct patients to drink a sufficient amount of water to ensure adequate hydration before their imaging and urge them to drink and urinate as often as possible during the first hours following the administration of Technetium Tc 99m Succimer Injection, in order to reduce radiation exposure [see Dosage and Administration (2.2) and Warnings and Precautions (5.2)].
Lactation
Advise a lactating woman to temporarily discontinue breastfeeding and to pump and discard breast milk for at least 24 hours after Technetium Tc 99m Succimer Injection administration in order to minimize radiation exposure to a breastfed infant [see Use in Specific Populations (8.2)].
PRINCIPAL DISPLAY PANEL - 1 mg Vial Carton
NEPHROSCAN®
Kit for the Preparation of Technetium Tc 99m Succimer Injection
1 mg per vial Succimer
For intravenous use only after radiolabeling with
Sodium Pertechnetate Tc 99m Injection, USP
THERAGNOSTICS
NDC:71083-0020-5
Rx Only
5 Single-Dose Vials. Discard Unused Portion
| NEPHROSCAN
succimer injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Theragnostics Inc (080437847) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ROTOP Pharmaka GmbH | 314666202 | analysis(71083-0020) , api manufacture(71083-0020) , label(71083-0020) , manufacture(71083-0020) , pack(71083-0020) | |
More about Nephroscan (succimer)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
