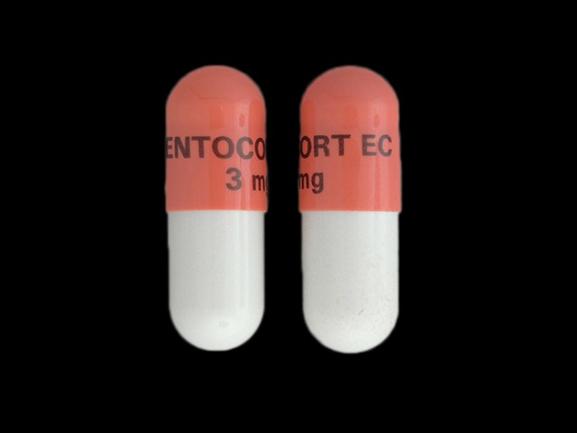
ENTOCORT EC 3mg Pill: pink gray, capsule/oblong, 7mm
The pill with imprint ENTOCORT EC 3mg (Pink / Gray, Capsule/Oblong, 7mm) has been identified as Budesonide (Enteric Coated) 3 mg and is used for Inflammatory Bowel Disease, Crohn's Disease, Eosinophilic Esophagitis, Asthma, Maintenance, and Asthma. It belongs to the drug classes glucocorticoids, inhaled corticosteroids and is not a controlled substance.
Images for ENTOCORT EC 3mg
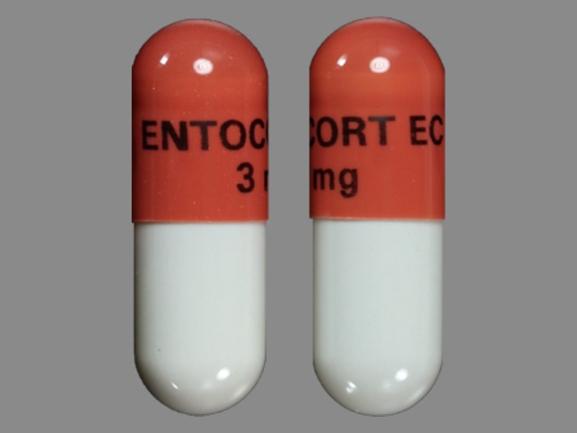
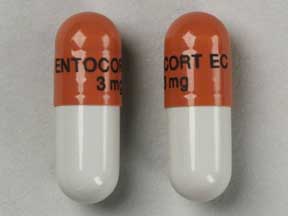
Budesonide (Enteric Coated)
- Imprint
- ENTOCORT EC 3mg
- Strength
- 3 mg
- Color
- Pink / Gray
- Size
- 7.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- Glucocorticoids, Inhaled corticosteroids
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Par Pharmaceutical Inc.
- Inactive Ingredients
-
ethylcellulose,
acetyltributyl citrate,
methacrylic acid,
triethyl citrate,
polysorbate 80,
magnesium silicate,
gelatin,
ferrosoferric oxide,
titanium dioxide,
ferric oxide red,
ferric oxide yellow,
sucrose
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00186-0702 (Discontinued) | AstraZeneca |
| 00574-9850 (Discontinued) | Paddock Laboratories, Inc. |
| 49884-0501 (Discontinued) | Par Pharmaceutical, Inc. |
| 54868-4910 (Discontinued) | Physicians Total Care Inc. (repackager) |
Related images for "ENTOCORT EC 3mg"
More about budesonide
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (188)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Support group
- Drug class: glucocorticoids
- Breastfeeding
Patient resources
Other brands
Pulmicort Flexhaler, Eohilia, Pulmicort Turbuhaler, Entocort EC, ... +4 more
Professional resources
- Budesonide (Systemic, Oral Inhalation) monograph
- Budesonide Capsule (FDA)
- Budesonide Capsule Delayed Release (FDA)
- Budesonide ER Tablets (FDA)
- Budesonide Inhalation Suspension (FDA)
- Budesonide Rectal Foam (FDA)
Other brands
Pulmicort Flexhaler, Eohilia, Pulmicort Turbuhaler, Entocort EC, ... +3 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.