Rotateq Dosage
Generic name: HUMAN ROTAVIRUS A TYPE G1P7(5) STRAIN WI79 LIVE ANTIGEN 2200000[iU] in 2mL, HUMAN ROTAVIRUS A TYPE G2P7(5) STRAIN SC2 LIVE ANTIGEN 2800000[iU] in 2mL, HUMAN ROTAVIRUS A TYPE G3P7(5) STRAIN WI78 LIVE ANTIGEN 2200000[iU] in 2mL, HUMAN ROTAVIRUS A TYPE G4P7(5) STRAIN BRB LIVE ANTIGEN 2000000[iU] in 2mL, HUMAN ROTAVIRUS A TYPE G6P1A(8) STRAIN WI79 LIVE ANTIGEN 2300000[iU] in 2mL
Dosage form: oral, pentavalent solution
Drug class: Viral vaccines
Medically reviewed by Drugs.com. Last updated on Nov 1, 2024.
FOR ORAL USE ONLY. NOT FOR INJECTION.
The vaccination series consists of three ready-to-use liquid doses of RotaTeq administered orally starting at 6 to 12 weeks of age, with the subsequent doses administered at 4- to 10-week intervals. The third dose should not be given after 32 weeks of age.
There are no restrictions on the infant's consumption of food or liquid, including breast milk, either before or after vaccination with RotaTeq.
Do not mix the RotaTeq vaccine with any other vaccines or solutions. Do not reconstitute or dilute.
For storage instructions.
Each dose is supplied in a container consisting of a squeezable plastic dosing tube with a twist-off cap, allowing for direct oral administration. The dosing tube is contained in a pouch.
Use with Other Vaccines
In clinical trials, RotaTeq was administered concomitantly with other licensed pediatric vaccines.
Instructions for Use
| To administer the vaccine: | |
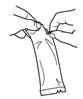 |
Tear open the pouch and remove the dosing tube. |
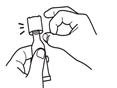 |
Clear the fluid from the dispensing tip by holding tube vertically and tapping cap. |
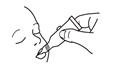
| Open the dosing tube in 2 easy motions: | |
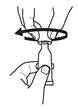 |
1. Puncture the dispensing tip by screwing cap clockwise until it becomes tight. |
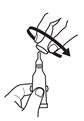 |
2. Remove cap by turning it counterclockwise. |
 |
Administer dose by gently squeezing liquid into infant's mouth toward the inner cheek until dosing tube is empty. (A residual drop may remain in the tip of the tube.) |
| If for any reason an incomplete dose is administered (e.g., infant spits or regurgitates the vaccine), a replacement dose is not recommended, since such dosing was not studied in the clinical trials. The infant should continue to receive any remaining doses in the recommended series. |
|
| Discard the empty tube and cap in approved biological waste containers according to local regulations. | |
More about RotaTeq (rotavirus vaccine)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- FDA approval history
- Drug class: viral vaccines
- En español
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.

