Flumist Quadrivalent Dosage
Generic name: INFLUENZA A VIRUS A/NORWAY/31694/2022 (H1N1) LIVE (ATTENUATED) ANTIGEN 10000000[FFU] in 0.2mL, INFLUENZA A VIRUS A/NORWAY/16606/2021 (H3N2) LIVE (ATTENUATED) ANTIGEN 10000000[FFU] in 0.2mL, INFLUENZA B VIRUS B/AUSTRIA/1359417/2021 LIVE (ATTENUATED) ANTIGEN 10000000[FFU] in 0.2mL, INFLUENZA B VIRUS B/PHUKET/3073/2013 LIVE (ATTENUATED) ANTIGEN 10000000[FFU] in 0.2mL
Dosage form: nasal spray
Drug class: Viral vaccines
Medically reviewed by Drugs.com. Last updated on Aug 4, 2025.
FOR INTRANASAL ADMINISTRATION BY A HEALTHCARE PROVIDER.
Dosing Information
Administer FluMist Quadrivalent according to the following schedule:
|
Age |
Dose |
Schedule |
|
2 years through 8 years |
If 2 doses, administer at least 1 month apart |
|
|
9 years through 49 years |
1 dose, 0.2 mL† |
- |
|
“-” indicates information is not applicable. |
||
Administration Instructions
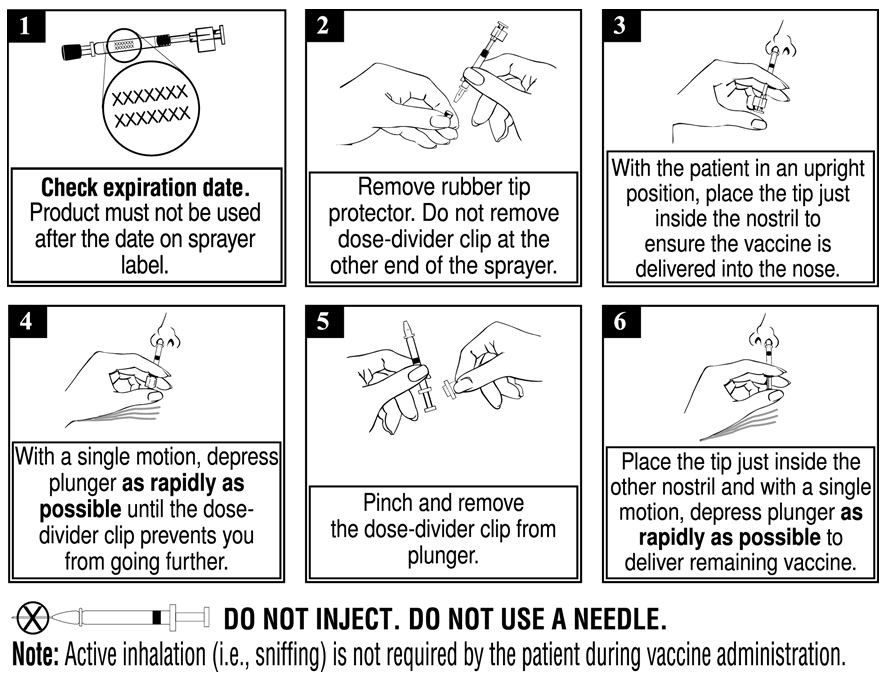
Each sprayer contains a single dose (0.2 mL) of FluMist Quadrivalent; administer approximately one half of the contents of the single-dose intranasal sprayer into each nostril (each sprayer contains 0.2 mL of vaccine). Refer to Figure 1 for step-by-step administration instructions. Following administration, dispose of the sprayer according to the standard procedures for medical waste (e.g., sharps container or biohazard container).
Figure 1
Frequently asked questions
- How and where is a flu shot injection given?
- What flu vaccine can I use with an egg allergy?
- Where can I get the flu vaccine right now?
- How well does the flu vaccine work?
- How can I get a flu vaccine without a needle?
More about FluMist Quadrivalent (influenza virus vaccine, live)
- Check interactions
- Compare alternatives
- Reviews (1)
- Drug images
- Side effects
- During pregnancy
- Drug class: viral vaccines
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.