Corifact Dosage
Generic name: Factor XIII Concentrate (Human) 1600[iU] in 20mL;
Dosage form: injection
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on Aug 8, 2025.
Dose
- 40 International Units (IU) per kg body weight at a rate not to exceed 4 mL per minute
- Adjust dose ±5 IU per kg to maintain 5% to 20% trough level of FXIII activity as provided in the example below
| FXIII Activity Trough Level (%) | Dosage Change |
|---|---|
| One trough level of <5% | Increase by 5 IU per kg |
| Trough level of 5% to 20% | No change |
| Two trough levels of >20% | Decrease by 5 IU per kg |
| One trough level of >25% | Decrease by 5 IU per kg |
Administration
- Administer at a rate not exceeding 4 mL per minute
- For routine prophylaxis, administer every 28 days
- For peri-operative management of surgical bleeding:
- Dosing should be individualized based on the patient's FXIII activity level, type of surgery, and clinical response
- Monitor patient's FXIII activity levels during and after surgery
- Following are dose adjustment examples for peri-operative management in reference to the patient's last prophylactic dose:
| Time Since Last Dose | Dose |
|---|---|
| Within 7 days | Additional dose may not be needed |
| 8 – 21 days | Additional partial or full dose may be needed based on FXIII activity level |
| 21 – 28 days | Full prophylactic dose |
The potency expressed in International Units is determined using the Berichrom activity assay, referenced to the current International Standard for Blood Coagulation Factor XIII, Plasma.
Reconstitution
Perform a visual inspection of the reconstituted solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
The procedures below are provided as general guidelines for the preparation and reconstitution of CORIFACT.
Reconstitute CORIFACT at room temperature as follows:
- Ensure that the CORIFACT vial and diluent vial are at room temperature.
- Place the CORIFACT vial, diluent vial, and Mix2Vial® transfer set on a flat surface.
- Remove CORIFACT and diluent vial flip caps. Wipe the stoppers with an alcohol swab and allow the stoppers to dry prior to opening the Mix2Vial transfer set package.
- Open the Mix2Vial transfer set package by peeling away the lid (Fig. 1). Leave the Mix2Vial transfer set in the clear package.
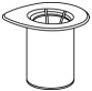
Fig. 1 - Place the diluent vial on a flat surface and hold the vial tightly. Grip the Mix2Vial transfer set together with the clear package and push the plastic spike at the blue end of the Mix2Vial transfer set firmly through the center of the stopper of the diluent vial (Fig. 2).
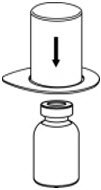
Fig. 2 - Carefully remove the clear package from the Mix2Vial transfer set. Make sure that you pull up only the clear package, not the Mix2Vial transfer set (Fig. 3).
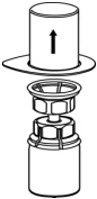
Fig. 3 - With the CORIFACT vial placed firmly on a flat surface, invert the diluent vial with the Mix2Vial transfer set attached and push the plastic spike of the transparent adapter firmly through the center of the stopper of the CORIFACT vial (Fig. 4). The diluent will automatically transfer into the CORIFACT vial.
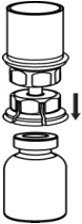
Fig. 4 - With the diluent and CORIFACT vial still attached to the Mix2Vial transfer set, gently swirl the CORIFACT vial to ensure that the CORIFACT is fully dissolved (Fig. 5). Do not shake the vial.
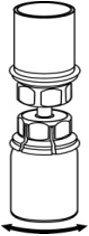
Fig. 5 - With one hand, grasp the CORIFACT side of the Mix2Vial transfer set and with the other hand grasp the blue diluent-side of the Mix2Vial transfer set, and unscrew the set into two pieces (Fig. 6).
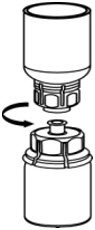
Fig. 6 - Draw air into an empty, sterile syringe. While the CORIFACT vial is upright, screw the syringe to the Mix2Vial transfer set. Inject air into the CORIFACT vial. While keeping the syringe plunger pressed, invert the system upside down and draw the concentrate into the syringe by pulling the plunger back slowly (Fig. 7).
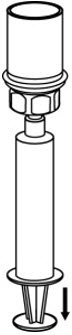
Fig. 7 - Now that the concentrate has been transferred into the syringe, firmly grasp the barrel of the syringe (keeping the plunger facing down) and unscrew the syringe from the Mix2Vial transfer set (Fig. 8). Attach the syringe to a suitable intravenous administration set.
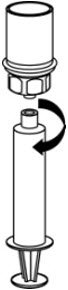
Fig. 8 - If patient requires more than one vial, pool the contents of multiple vials into one syringe. Use a separate unused Mix2Vial transfer set for each product vial.
- CORIFACT is for dose use only. Contains no preservatives. The product must be used within 4 hours after reconstitution. Do not refrigerate or freeze the reconstituted solution. Discard partially used vials.
More about Corifact (factor XIII)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous coagulation modifiers
- En español
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.









