Brineura Dosage
Generic name: CERLIPONASE ALFA 150mg in 5mL;
Dosage form: kit
Drug class: Lysosomal enzymes
Medically reviewed by Drugs.com. Last updated on Aug 5, 2024.
Recommendations Prior to BRINEURA Treatment
- Administration of BRINEURA should be supervised by a healthcare provider knowledgeable in the management of hypersensitivity reactions including anaphylaxis.
- Initiate BRINEURA in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment.
- BRINEURA should be administered by or under the supervision of a physician experienced in intraventricular administration via a surgically implanted intraventricular access device system which consists of the reservoir and catheter components.
- Premedication of patients with antihistamines with or without antipyretics or corticosteroids is recommended 30 to 60 minutes prior to the start of infusion.
- Aseptic technique must be strictly observed during preparation and administration.
- Prior to each infusion of BRINEURA, inspect the scalp for signs of intraventricular access device reservoir leakage, failure or potential infection.
- Prior to each infusion of BRINEURA and when clinically indicated, obtain a sample of CSF for cell count and culture.
- Replace the intraventricular access device reservoir prior to 4 years of single-puncture administrations.
Recommended Dosage and Administration
The recommended dosage of BRINEURA in pediatric patients is provided in Table 1. The dose is administered once every other week by intraventricular infusion.
BRINEURA is not recommended in patients less than 37 weeks post-menstrual age (gestational age at birth plus post-natal age) or those weighing less than 2.5 kg.
Administer BRINEURA first followed by infusion of the Intraventricular Electrolytes. The complete BRINEURA infusion, including the required infusion of Intraventricular Electrolytes, is approximately 2 to 4.5 hours, depending on the dose and volume administered. See Table 1 for the appropriate volume and infusion rate. For volumes that are not whole numbers, see Dosage and Administration (2.6).
| Age groups | BRINEURA dose administered every other week | Volume of BRINEURA solution | Infusion rate |
|---|---|---|---|
| Birth to < 6 months | 100 mg | 3.3 mL | 1.25 mL/hr |
| 6 months to < 1 year | 150 mg | 5 mL | 2.5 mL/hr |
| 1 year to < 2 years | 200 mg (first 4 doses) | 6.7 mL (first 4 doses) | 2.5 mL/hr |
| | 300 mg (subsequent doses) | 10 mL (subsequent doses) | |
| 2 years and older | 300 mg | 10 mL | 2.5 mL/hr |
Administration Modifications due to Hypersensitivity Reactions Including Anaphylaxis
In the event of a severe hypersensitivity reaction (e.g., anaphylaxis), immediately discontinue BRINEURA administration and initiate appropriate medical treatment. If the decision is made to readminister BRINEURA after the occurrence of anaphylaxis, initiate the subsequent infusion at approximately one-half the infusion rate at which the anaphylactic reaction occurred with appropriate premedication. For additional recommendations in the event of a hypersensitivity reaction, see Warnings and Precautions (5.1).
Preparation Instructions for Intraventricular Administration of the Product
BRINEURA and the Intraventricular Electrolytes must only be administered by the intraventricular route, using the provided Administration Kit for use with BRINEURA. Each vial of BRINEURA and Intraventricular Electrolytes is intended for a single dose only.
BRINEURA is administered into the cerebrospinal fluid (CSF) by infusion via a surgically implanted reservoir and catheter (the "intraventricular access device system"). BRINEURA is intended to be administered via the Codman® HOLTER RICKHAM Reservoirs (Part Numbers: 82-1625, 82-1621, 82-1616) with the Codman® Ventricular Catheter (Part Number: 82-1650). The intraventricular access device reservoir and catheter must be implanted prior to the first infusion. It is recommended that the first dose be administered at least 5 to 7 days after device implantation.
BRINEURA is intended to be administered with the B Braun Perfusor® Space Infusion Pump System (Product Code: 8713030U). If an alternative pump must be used, the essential performance requirements for this syringe pump used to deliver BRINEURA are as follows:
- Delivery rate of 1.25 or 2.5 mL per hr with delivery accuracy of +/- 1 mL per hr
- Compatible with 20 mL syringes provided in the Administration Kit for use with BRINEURA
- Occlusion alarm setting to ≤ 281 mm Hg
- Cleared for intraventricular route of administration
Administer BRINEURA and the Intraventricular Electrolytes using the provided Administration Kit for use with BRINEURA components.
Each infusion consists of 3.3 to 10 mL of BRINEURA followed by 2 mL of Intraventricular Electrolytes. The complete infusion must be administered using an infusion set with a 0.2 micron inline filter. The Intraventricular Electrolytes are used to flush the infusion line, port needle, and intraventricular access device in order to fully administer BRINEURA and to maintain patency of the intraventricular access device.
Supplies for Infusion Preparation
- BRINEURA and Intraventricular Electrolytes Injection vials (package 1 of 2)
- Administration Kit for use with BRINEURA (package 2 of 2)
- Syringe pump (not supplied)
- Empty sterile single-use luer lock syringe, no larger than 3 mL (not supplied)
Inspect the Administration Kit infusion components to ensure the components are in the individual packages and have not been compromised.
Thaw BRINEURA and Intraventricular Electrolytes Injection vials at room temperature 20°C to 25°C (68°F to 77°F) for approximately 60 minutes. Do not thaw or warm vials any other way. Do not shake vials. Condensation will occur during thawing period. Do not re-freeze vials or freeze syringes containing BRINEURA or Intraventricular Electrolytes.
Inspect fully thawed BRINEURA and Intraventricular Electrolytes Injection vials. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. BRINEURA is a clear to slightly opalescent and colorless to pale yellow solution. Intraventricular Electrolytes is a clear to colorless solution. Do not use if the solutions are discolored or if there is other foreign particulate matter in the solutions. BRINEURA vials may occasionally contain thin translucent fibers or opaque particles. These naturally occurring particles are cerliponase alfa. These particles are removed via the 0.2 micron inline filter without having a detectable effect on the purity or strength of BRINEURA. Intraventricular Electrolytes may contain particles, which appear during the thaw period; however, these dissolve when the solution reaches room temperature.
Storage Instructions for the Thawed Product and Product in Syringe
Storage of Thawed Product
Use thawed BRINEURA and Intraventricular Electrolytes immediately. If thawed BRINEURA and Intraventricular Electrolytes is not used immediately, store in the refrigerator at 2°C to 8°C (36°F to 46°F) for no more than 24 hours. Discard the thawed product after 24 hours if refrigerated.
Storage of Product in Syringe
Use product held in labeled syringes immediately. If product held in labeled syringe is not used immediately, store in the refrigerator at 2°C to 8°C (36°F to 46°F) for no more than 4 hours prior to infusion. Discard the product held in labeled syringe after 4 hours if refrigerated.
Administration Instructions for Intraventricular Infusion
Intraventricular Infusion of BRINEURA
Figure 1 represents the intraventricular infusion system set up. Use aseptic technique during the infusion.
Follow the steps below to proceed with the intraventricular infusion.
 |
| Figure 1 |
- 1.
- Use aseptic technique when preparing the BRINEURA syringe for infusion. Label one sterile syringe "BRINEURA" and attach the syringe needle.
- 2.
- Confirm required dose and volume based on patient age per Table 1. Remove the green flip-off caps from one or both BRINEURA vials. Each BRINEURA vial contains 150 mg or 5 mL. Use the "BRINEURA" labeled syringe to withdraw the volume of BRINEURA solution from the vial per the required dose (see Table 1). Intermediate volumes that fall between 1 mL increments should be drawn up in the syringe to the nearest whole number, specifically 3.3 mL to 4 mL and 6.7 mL to 7 mL. Do not dilute BRINEURA. Do not mix BRINEURA with any other drug.
Discard any unused portion left in the vial. - 3.
- Label the infusion line "intraventricular infusion only" (see Figure 1).
- 4.
- Attach the syringe containing BRINEURA to the extension line (see Figure 2). Then connect the extension line to the infusion set with a 0.2 micron inline filter (see Figure 1).
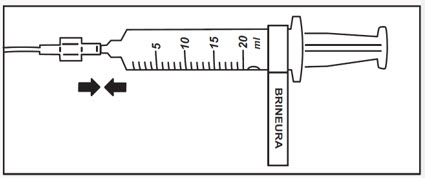
Figure 2 - 5.
- Prime the infusion components with BRINEURA.
- 6.
- Inspect scalp for signs of intraventricular access device reservoir leakage or failure and for potential infections.
- 7.
- Prepare the scalp for intraventricular infusion per institution standard of care.
- 8.
- Insert port needle into intraventricular access device reservoir (see Figure 3).
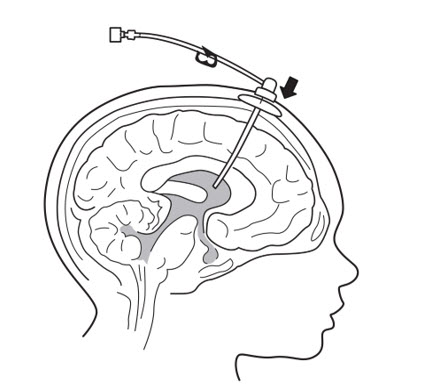
Figure 3 - 9.
- Connect a separate empty sterile single-use luer lock syringe, no larger than 3 mL (not provided) to the port needle. Withdraw 0.5 mL to 1 mL of CSF to check patency of intraventricular access device (see Figure 4) and send specimen for culture.
- Do not return CSF to intraventricular access device.
- Routinely send CSF samples for infection monitoring.
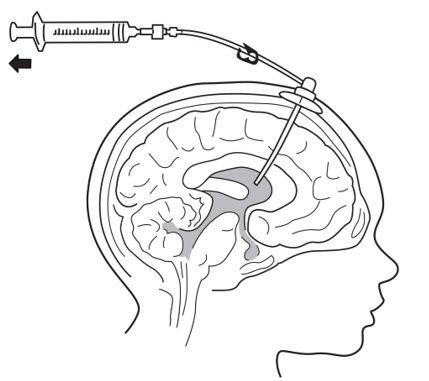
Figure 4 - 10.
- Attach the infusion set with 0.2 micron inline filter to the port needle (see Figure 1).
- Secure the components per institution standard of care.
- 11.
- Place the syringe containing BRINEURA into the syringe pump and program the pump to deliver at an infusion rate of 1.25 or 2.5 mL per hour (Table 1). Set the pump volume limit to deliver the exact volume that corresponds to the dose of BRINEURA solution appropriate for the patient's age (Table 1). Set the occlusion alarm setting to alert at pressure ≤ 281 mm Hg. See syringe pump operating manual for details. Do not deliver as a bolus or manually.
- 12.
- Administer pre-medication 30 to 60 minutes prior to the start of infusion.
- 13.
- Monitor vital signs (blood pressure, heart rate) prior to the start of infusion, periodically during infusion, and post-infusion.
- 14.
- Initiate infusion of BRINEURA at a rate of 1.25 or 2.5 mL per hour (Table 1).
- 15.
- Periodically inspect the infusion system during the infusion for signs of leakage or delivery failure.
- 16.
- When the BRINEURA infusion is complete, detach and remove the syringe from the pump and disconnect from the tubing (see Figure 5). Discard the syringe containing any residual drug. Proceed to Step 16 for Intraventricular Electrolytes infusion.
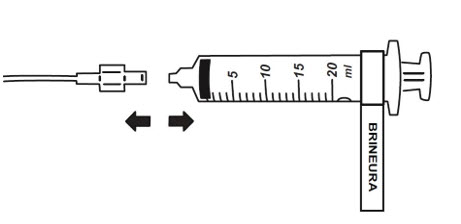
Figure 5
Intraventricular Infusion of Intraventricular Electrolytes
Administer the Intraventricular Electrolytes provided after BRINEURA infusion is complete.
- 17.
- Use aseptic technique when preparing the Intraventricular Electrolytes syringe for infusion. Label one sterile syringe "Intraventricular Electrolytes" and attach the syringe needle. Remove the yellow flip-off cap from the Intraventricular Electrolytes Injection vial. Withdraw 2 mL of Intraventricular Electrolytes. Discard the remaining unused portion.
- 18.
- Attach the syringe to the extension line (see Figure 6).
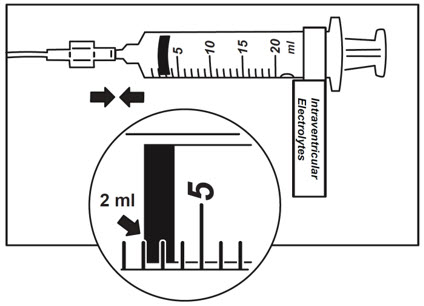
Figure 6 - 19.
- Place the syringe containing Intraventricular Electrolytes into the syringe pump and program pump to deliver at an infusion rate of 1.25 or 2.5 mL per hour (Table 1). Set the pump volume limit to deliver 2 mL. Set the occlusion alarm setting to alert at pressure ≤ 281 mm Hg. See syringe pump operating manual for details. Do not deliver as a bolus or manually.
- 20.
- Initiate infusion of Intraventricular Electrolytes at a rate of 2.5 mL per hour.
- 21.
- Periodically inspect the infusion system during the infusion for signs of leakage or delivery failure.
- 22.
- When the Intraventricular Electrolytes infusion is complete, detach and remove the empty syringe from the pump and disconnect from the infusion line.
- 23.
- Remove the port needle. Apply gentle pressure and bandage the infusion site per institution standard of care.
Dispose of the infusion components, needles, unused solutions and other waste materials in accordance with local requirements.
Frequently asked questions
More about Brineura (cerliponase alfa)
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- FDA approval history
- Drug class: lysosomal enzymes
- En español
Patient resources
Professional resources
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.






